Summary information and primary citation
- PDB-id
-
9c68;
SNAP-derived features in text and
JSON formats
- Class
- antiviral protein-RNA
- Method
- X-ray (1.82 Å)
- Summary
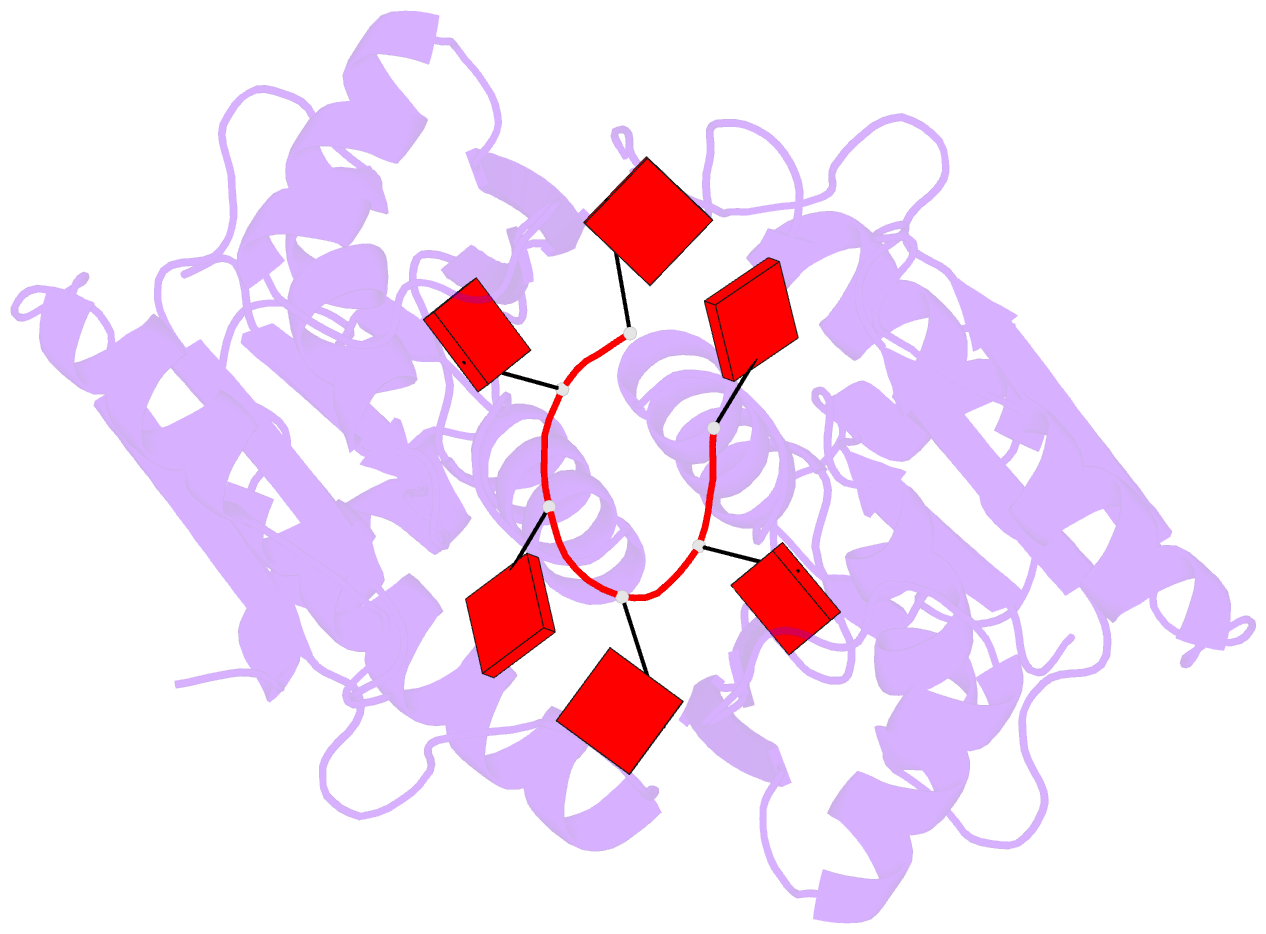
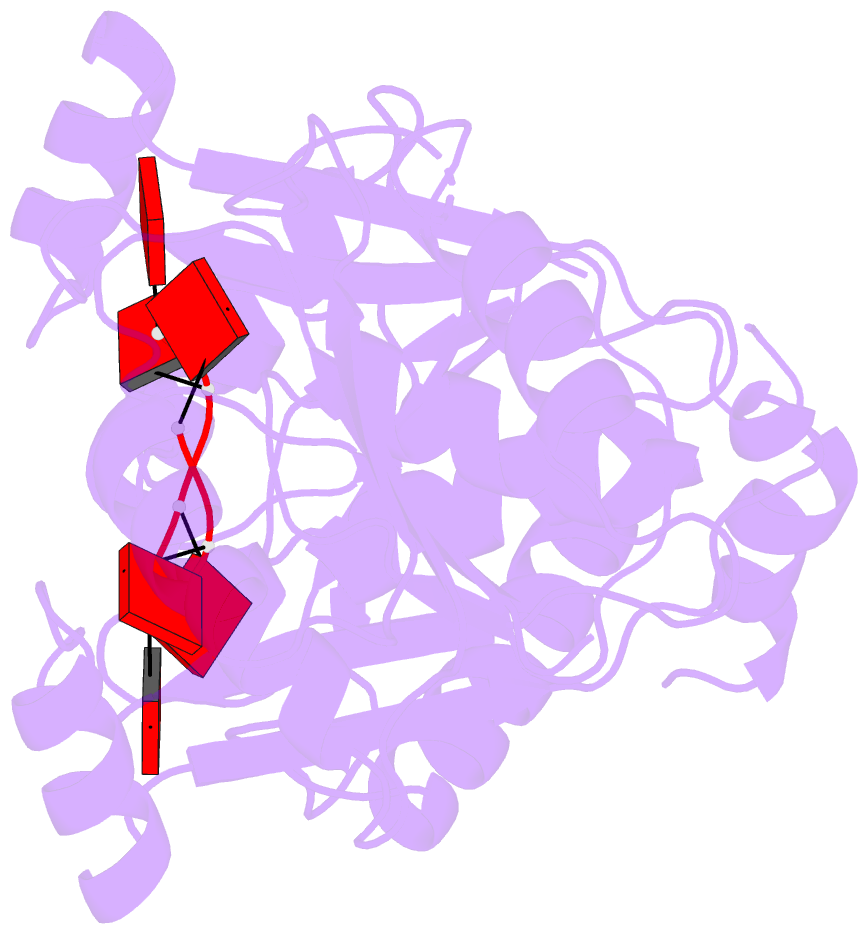
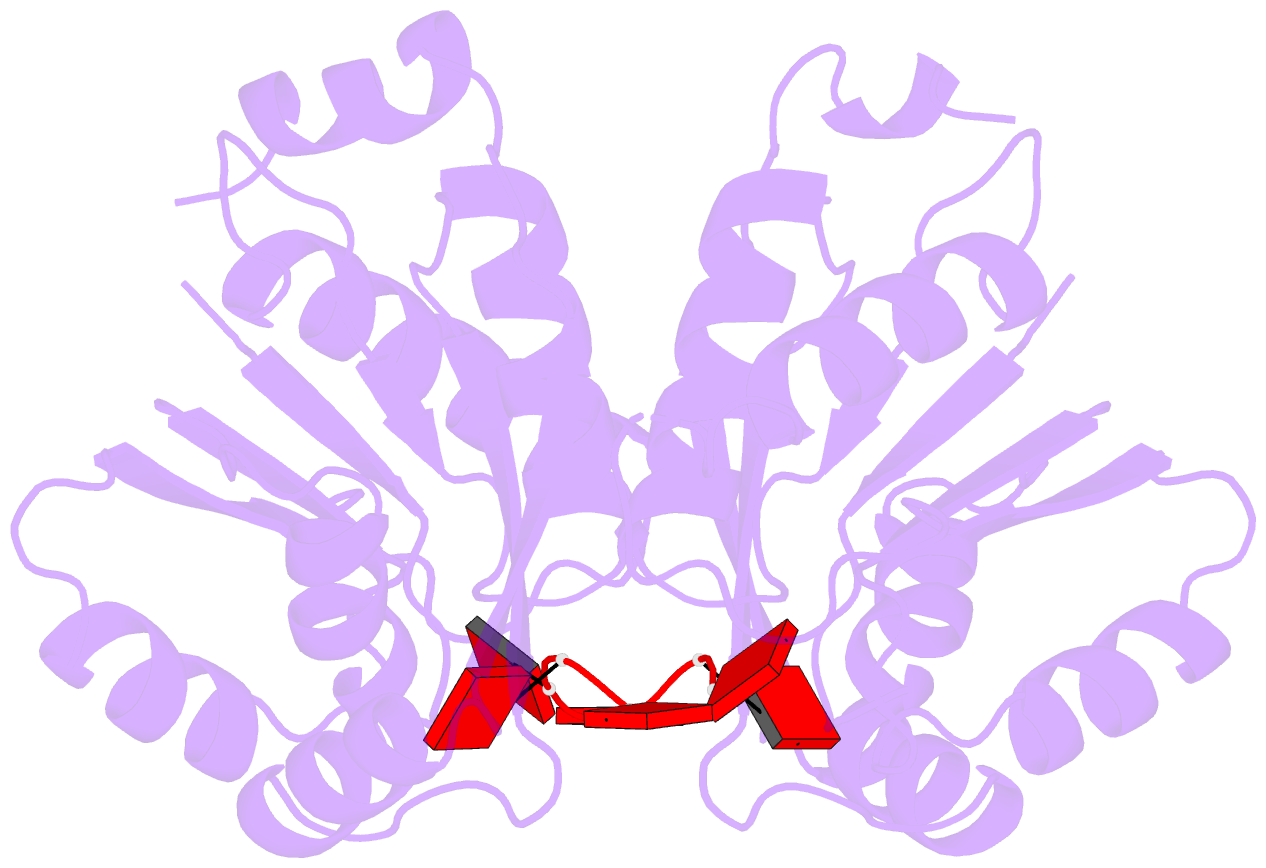
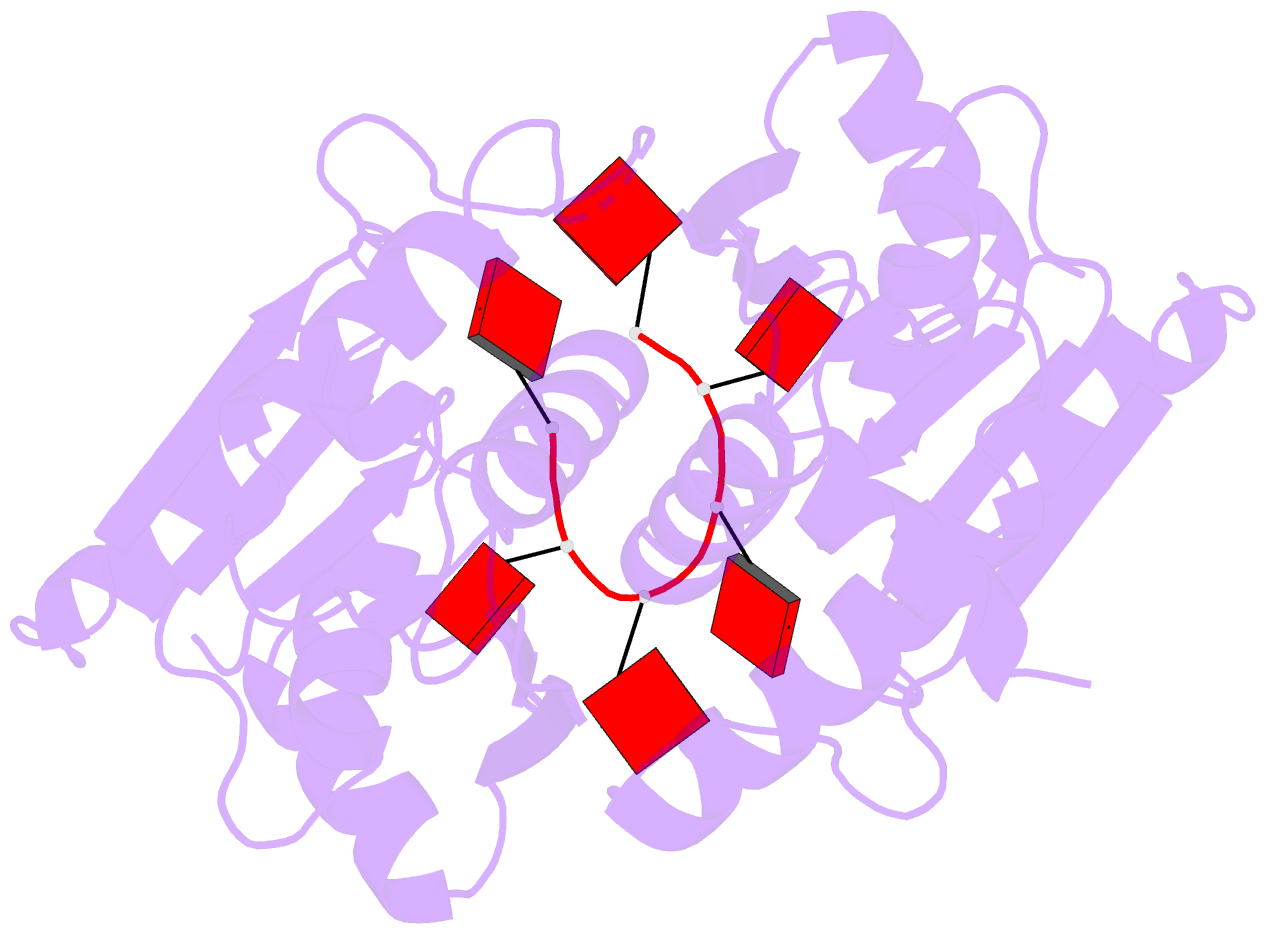
- The crispr associated carf-adenosine deaminase
cad1-carf in the ca6 bound form
- Reference
-
Baca CF, Majumder P, Hickling JH, Ye L, Teplova M, Brady
SF, Patel DJ, Marraffini LA (2024): "The
CRISPR-associated adenosine deaminase Cad1 converts ATP
to ITP to provide antiviral immunity." Cell.
doi: 10.1016/j.cell.2024.10.002.
- Abstract
- Type III CRISPR systems provide immunity against
genetic invaders through the production of cyclic
oligo-adenylate (cA<sub>n</sub>) molecules that
activate effector proteins that contain CRISPR-associated
Rossman fold (CARF) domains. Here, we characterized the
function and structure of an effector in which the CARF
domain is fused to an adenosine deaminase domain,
CRISPR-associated adenosine deaminase 1 (Cad1). We show
that upon binding of cA<sub>4</sub> or
cA<sub>6</sub> to its CARF domain, Cad1
converts ATP to ITP, both in vivo and in vitro.
Cryoelectron microscopy (cryo-EM) structural studies on
full-length Cad1 reveal an hexameric assembly composed of a
trimer of dimers, with bound ATP at inter-domain sites
required for activity and ATP/ITP within deaminase active
sites. Upon synthesis of cA<sub>n</sub> during
phage infection, Cad1 activation leads to a growth arrest
of the host that prevents viral propagation. Our findings
reveal that CRISPR-Cas systems employ a wide range of
molecular mechanisms beyond nucleic acid degradation to
provide adaptive immunity in prokaryotes.