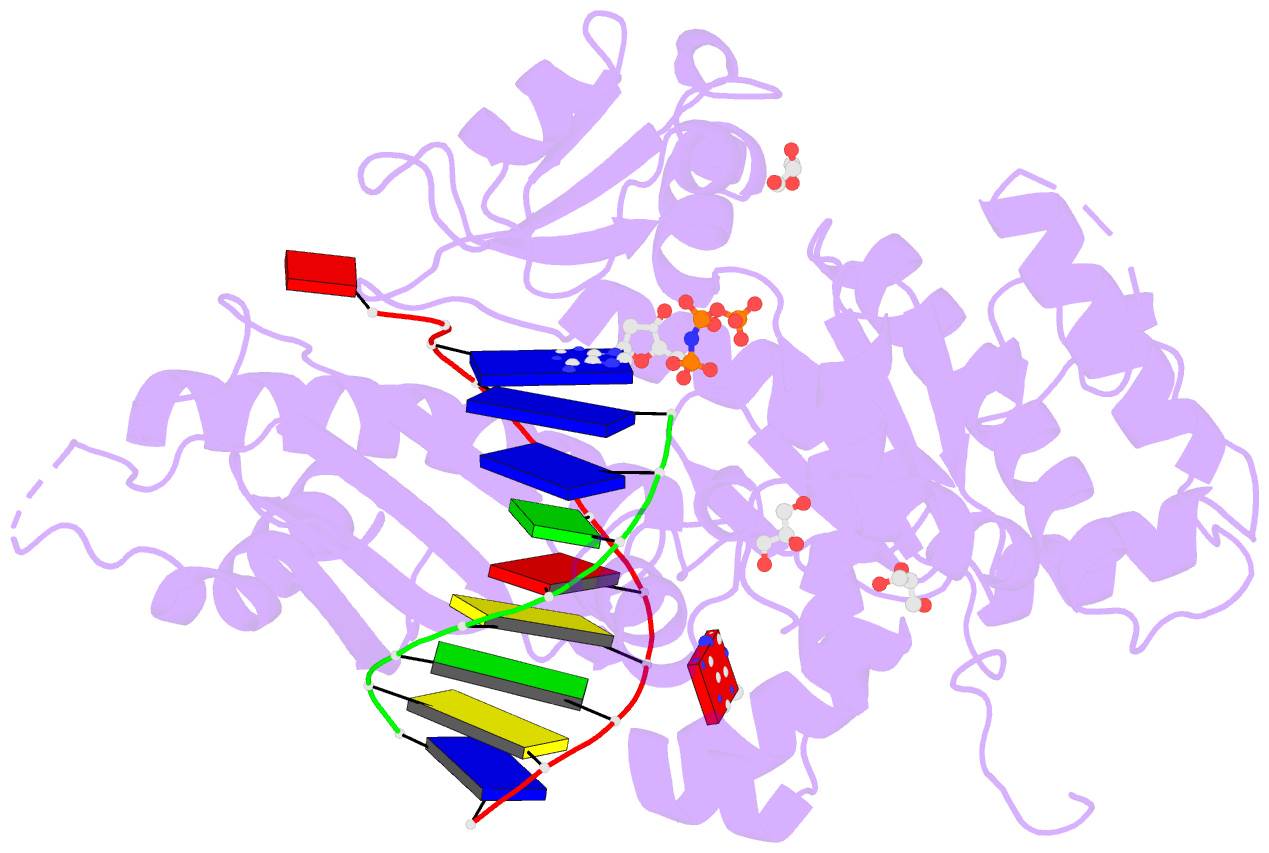
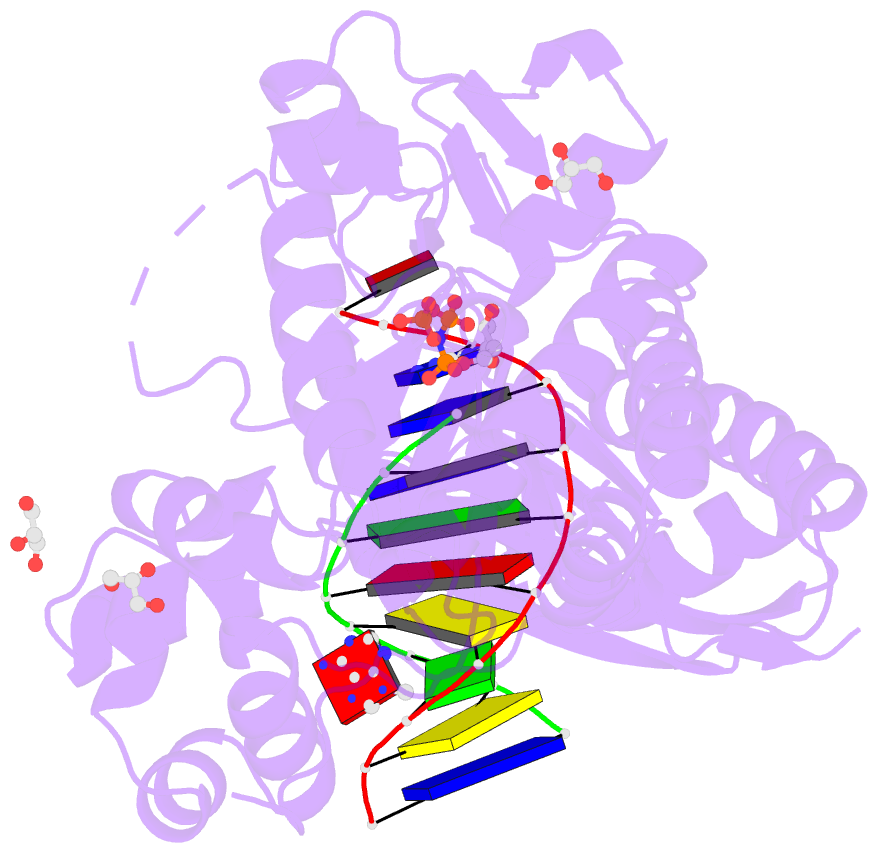
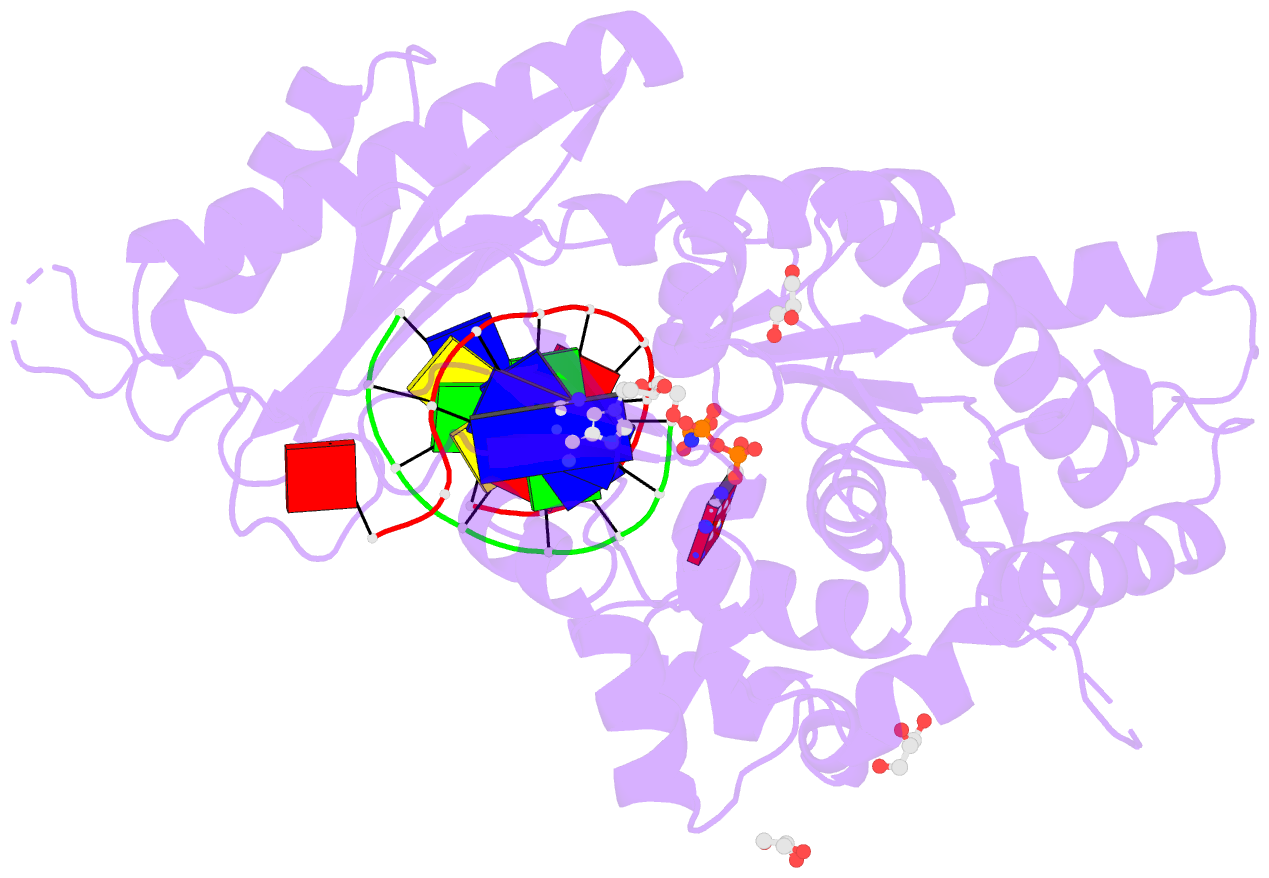
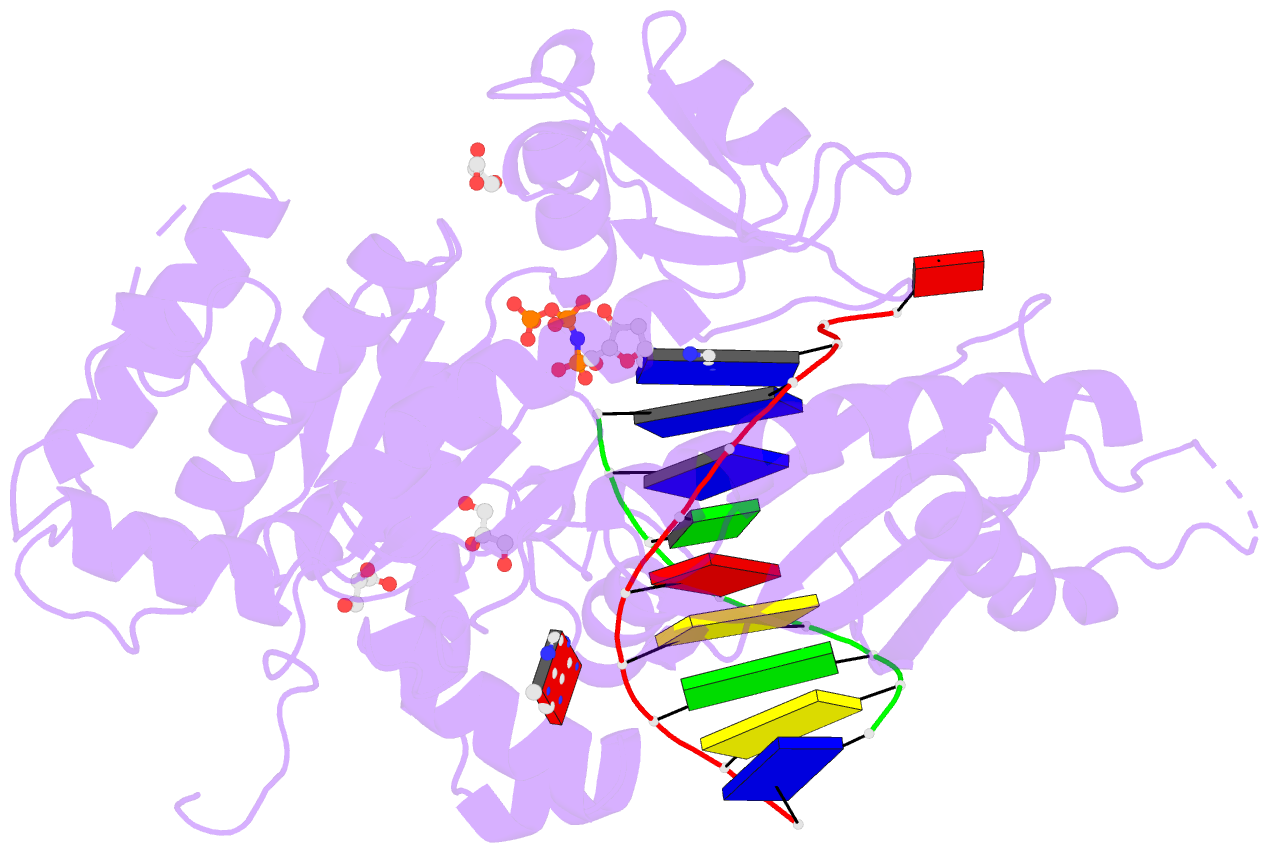
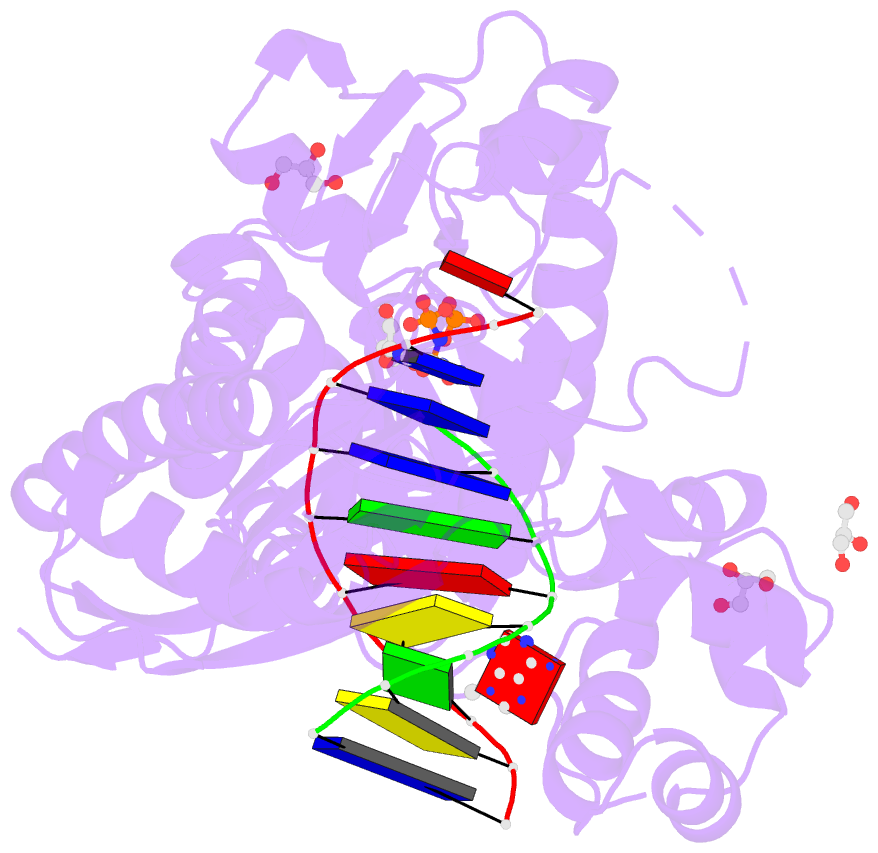
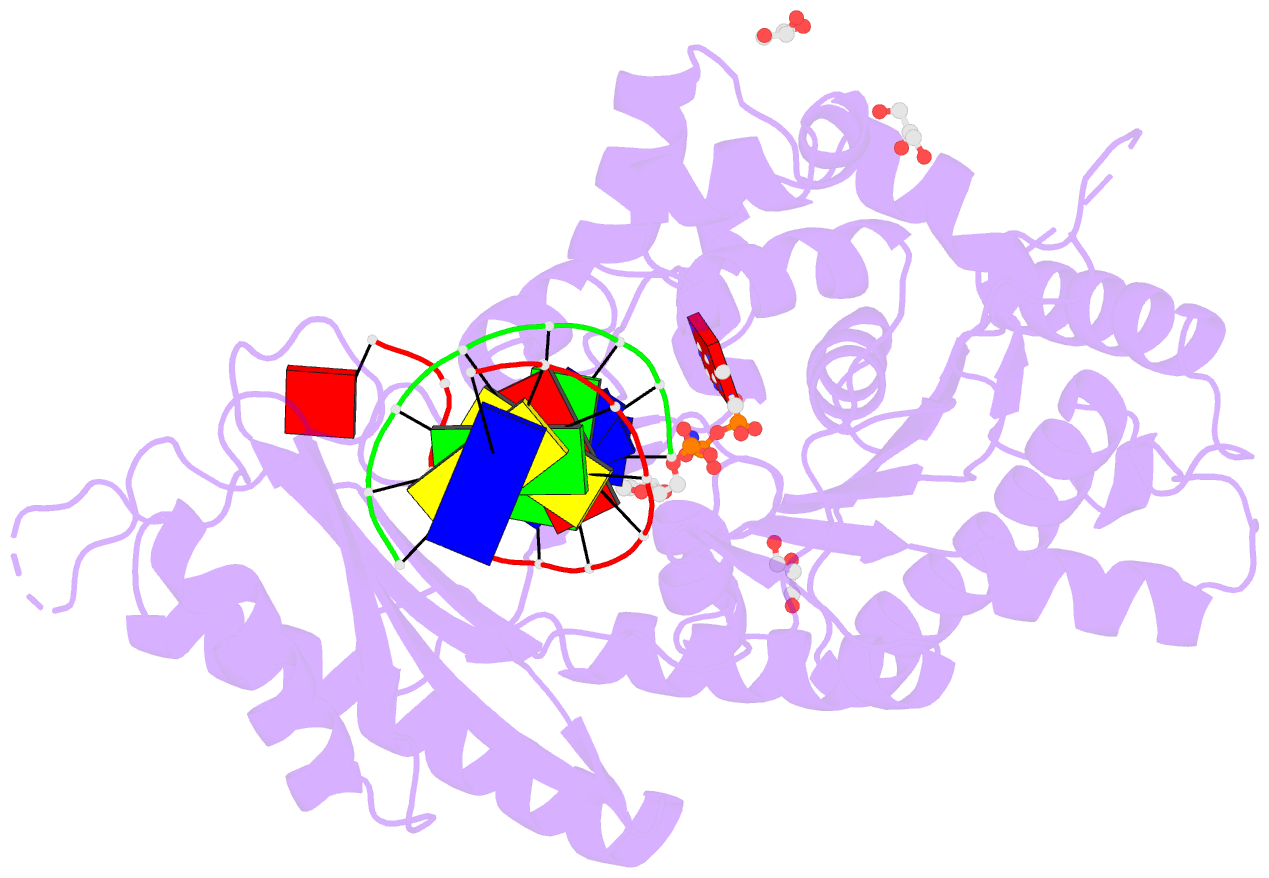
Summary information and primary citation
- PDB-id
-
7m7n;
SNAP-derived features in text and
JSON formats
- Class
- transferase-DNA
- Method
- X-ray (1.31 Å)
- Summary
- Human DNA pol eta with 2'-fa-ended primer and
dampnpp
- Reference
-
Gregory MT, Gao Y, Cui Q, Yang W (2021): "Multiple
deprotonation paths of the nucleophile 3'-OH in the DNA
synthesis reaction." Proc.Natl.Acad.Sci.USA,
118. doi: 10.1073/pnas.2103990118.
- Abstract
- DNA synthesis by polymerases is essential for life.
Deprotonation of the nucleophile 3'-OH is thought to be the
obligatory first step in the DNA synthesis reaction. We
have examined each entity surrounding the nucleophile 3'-OH
in the reaction catalyzed by human DNA polymerase (Pol) η
and delineated the deprotonation process by combining
mutagenesis with steady-state kinetics, high-resolution
structures of in crystallo reactions, and molecular
dynamics simulations. The conserved S113 residue, which
forms a hydrogen bond with the primer 3'-OH in the ground
state, stabilizes the primer end in the active site.
Mutation of S113 to alanine destabilizes primer binding and
reduces the catalytic efficiency. Displacement of a water
molecule that is hydrogen bonded to the 3'-OH using the
2'-OH of a ribonucleotide or 2'-F has little effect on
catalysis. Moreover, combining the S113A mutation with 2'-F
replacement, which removes two potential hydrogen acceptors
of the 3'-OH, does not reduce the catalytic efficiency. We
conclude that the proton can leave the O3' via alternative
paths, supporting the hypothesis that binding of the third
Mg<sub>2+</sub> initiates the reaction by
breaking the α-β phosphodiester bond of an incoming
deoxyribonucleoside triphosphate (dNTP).