Summary information and primary citation
- PDB-id
-
7k98;
SNAP-derived features in text and
JSON formats
- Class
- ligase-RNA
- Method
- X-ray (2.19 Å)
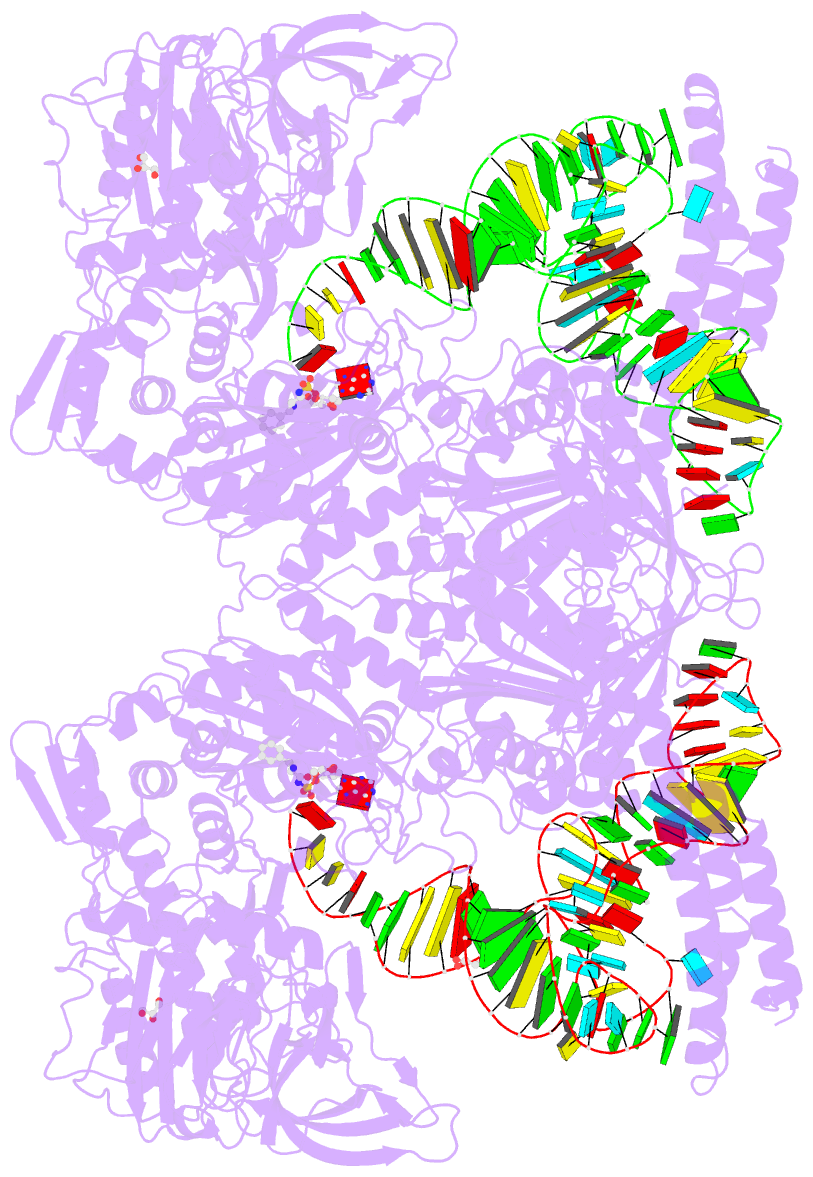
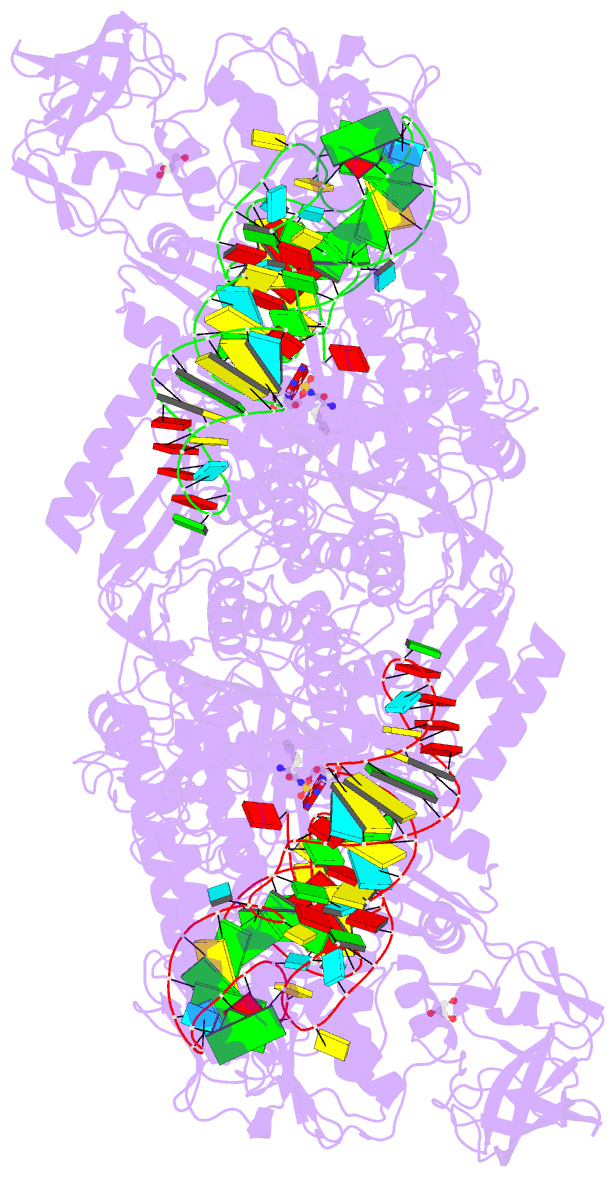
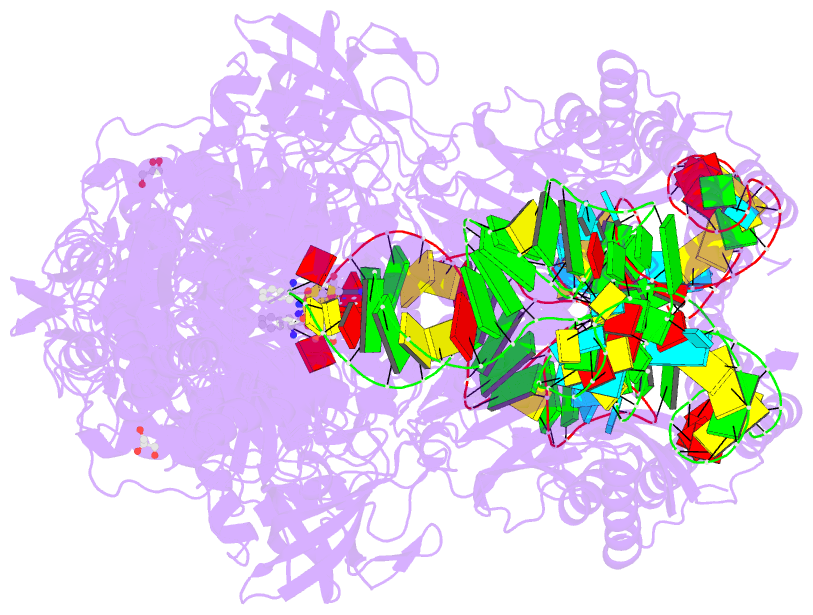
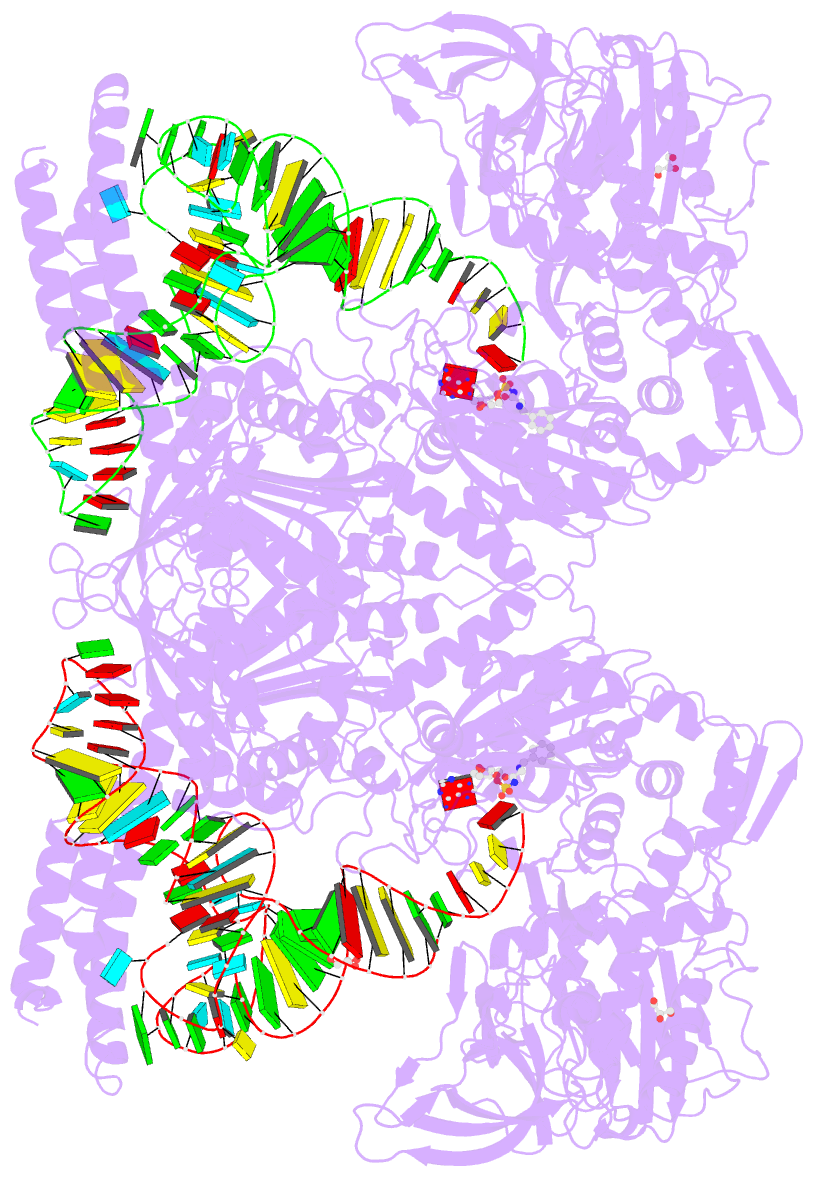
- Summary
- Preaminoacylation complex of m. tuberculosis phers with
cognate precursor trna and
5'-o-(n-phenylalanyl)sulfamoyl-adenosine (f-ams)
- Reference
-
Michalska K, Jedrzejczak R, Wower J, Chang C, Baragana B,
Gilbert IH, Forte B, Joachimiak A (2021): "Mycobacterium
tuberculosis Phe-tRNA synthetase: structural insights
into tRNA recognition and aminoacylation."
Nucleic Acids Res., 49,
5351-5368. doi: 10.1093/nar/gkab272.
- Abstract
- Tuberculosis, caused by Mycobacterium tuberculosis,
responsible for ∼1.5 million fatalities in 2018, is the
deadliest infectious disease. Global spread of multidrug
resistant strains is a public health threat, requiring new
treatments. Aminoacyl-tRNA synthetases are plausible
candidates as potential drug targets, because they play an
essential role in translating the DNA code into protein
sequence by attaching a specific amino acid to their
cognate tRNAs. We report structures of M. tuberculosis
Phe-tRNA synthetase complexed with an unmodified tRNAPhe
transcript and either L-Phe or a nonhydrolyzable
phenylalanine adenylate analog. High-resolution models
reveal details of two modes of tRNA interaction with the
enzyme: an initial recognition via indirect readout of
anticodon stem-loop and aminoacylation ready state
involving interactions of the 3' end of tRNAPhe with the
adenylate site. For the first time, we observe the protein
gate controlling access to the active site and detailed
geometry of the acyl donor and tRNA acceptor consistent
with accepted mechanism. We biochemically validated the
inhibitory potency of the adenylate analog and provide the
most complete view of the Phe-tRNA synthetase/tRNAPhe
system to date. The presented topography of amino
adenylate-binding and editing sites at different stages of
tRNA binding to the enzyme provide insights for the
rational design of anti-tuberculosis drugs.