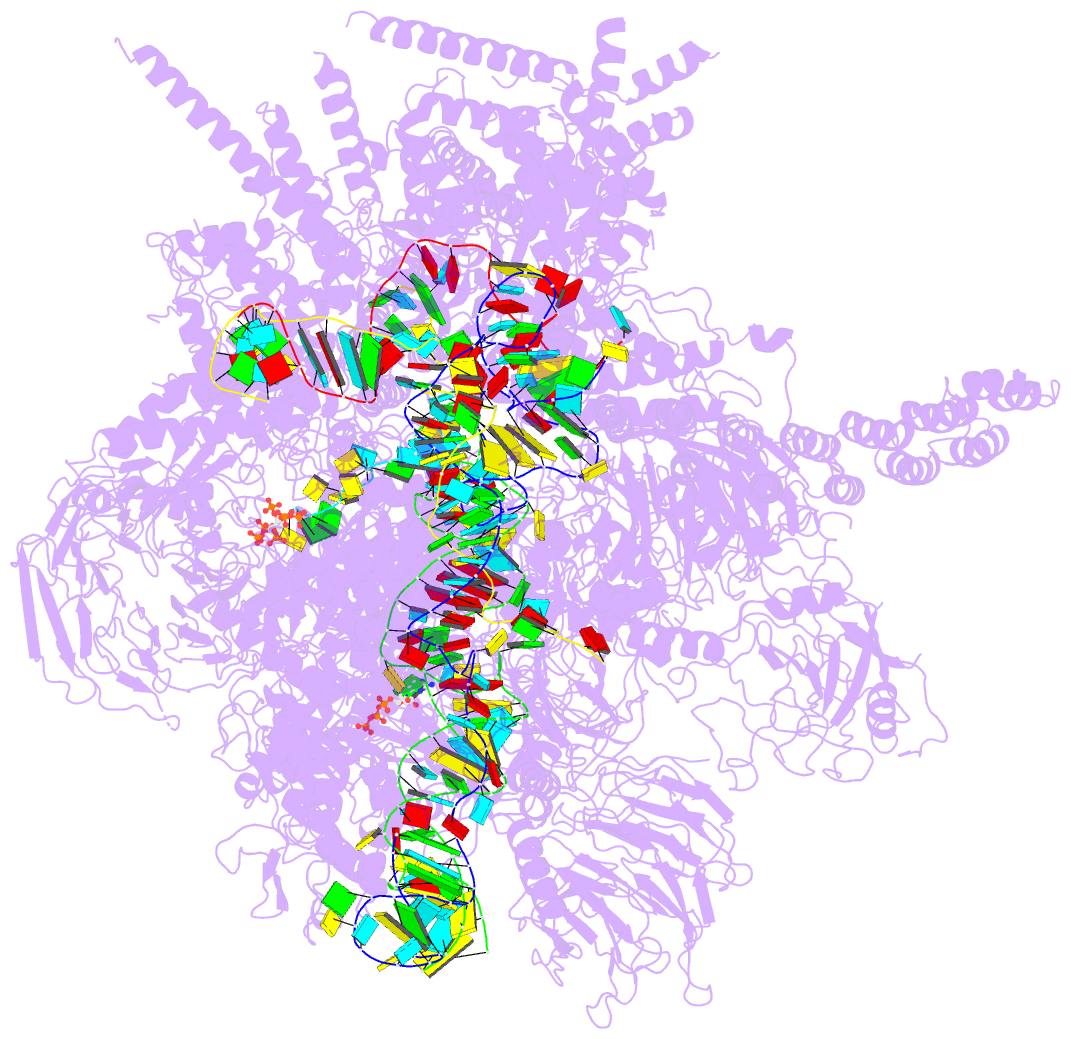
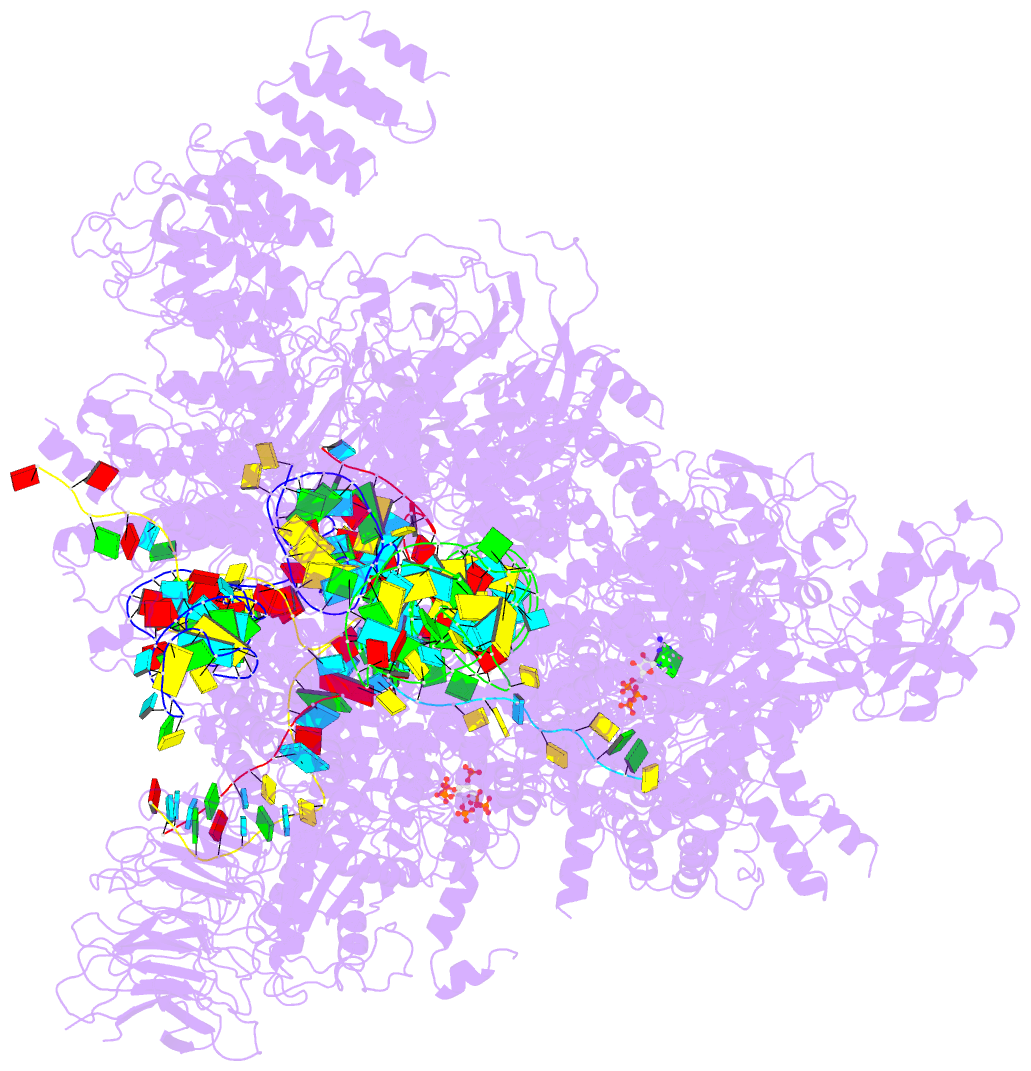
Summary information and primary citation
- PDB-id
-
6zym;
SNAP-derived features in text and
JSON formats
- Class
- splicing
- Method
- cryo-EM (3.4 Å)
- Summary
- Human c complex spliceosome - high-resolution core
- Reference
-
Bertram K, El Ayoubi L, Dybkov O, Agafonov DE, Will CL,
Hartmuth K, Urlaub H, Kastner B, Stark H, Luhrmann R
(2020): "Structural
Insights into the Roles of Metazoan-Specific Splicing
Factors in the Human Step 1 Spliceosome."
Mol.Cell, 80, 127-139.e6. doi:
10.1016/j.molcel.2020.09.012.
- Abstract
- Human spliceosomes contain numerous proteins absent in
yeast, whose functions remain largely unknown. Here we
report a 3D cryo-EM structure of the human spliceosomal C
complex at 3.4 Å core resolution and 4.5-5.7 Å at
its periphery, and aided by protein crosslinking we
determine its molecular architecture. Our structure
provides additional insights into the spliceosome's
architecture between the catalytic steps of splicing, and
how proteins aid formation of the spliceosome's
catalytically active RNP (ribonucleoprotein) conformation.
It reveals the spatial organization of the
metazoan-specific proteins PPWD1, WDR70, FRG1, and CIR1 in
human C complexes, indicating they stabilize functionally
important protein domains and RNA structures
rearranged/repositioned during the
B<sub>act</sub> to C transition. Structural
comparisons with human B<sub>act</sub>,
C<sub>∗</sub>, and P complexes reveal an
intricate cascade of RNP rearrangements during splicing
catalysis, with intermediate RNP conformations not found in
yeast, and additionally elucidate the structural basis for
the sequential recruitment of metazoan-specific
spliceosomal proteins.