Summary information and primary citation
- PDB-id
-
6zdp;
SNAP-derived features in text and
JSON formats
- Class
- replication
- Method
- X-ray (2.85 Å)
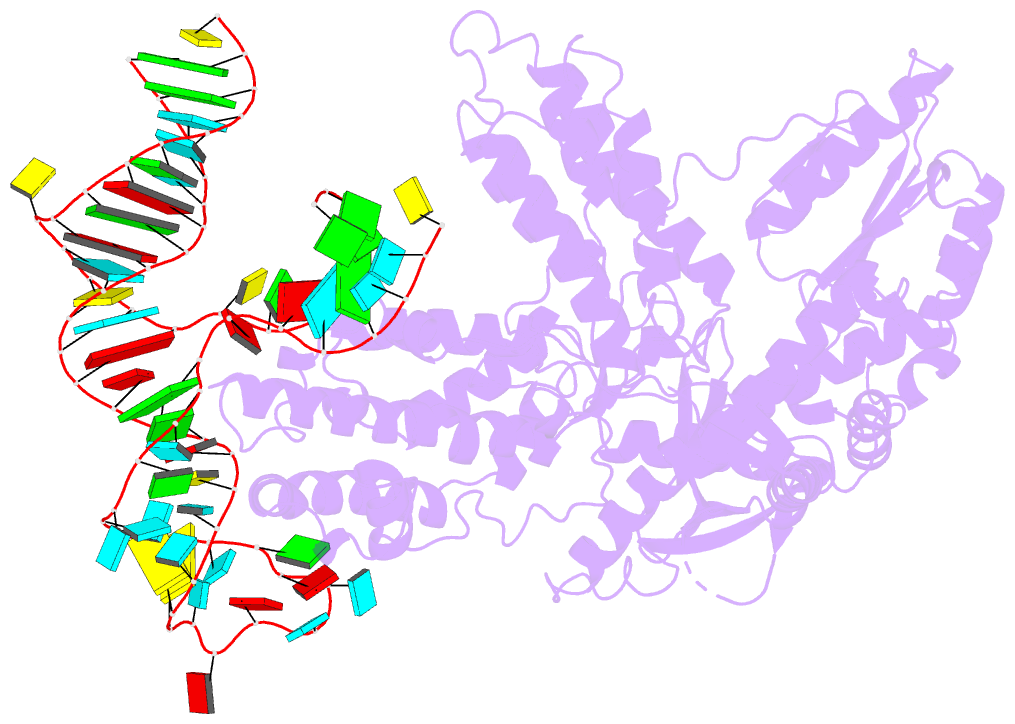
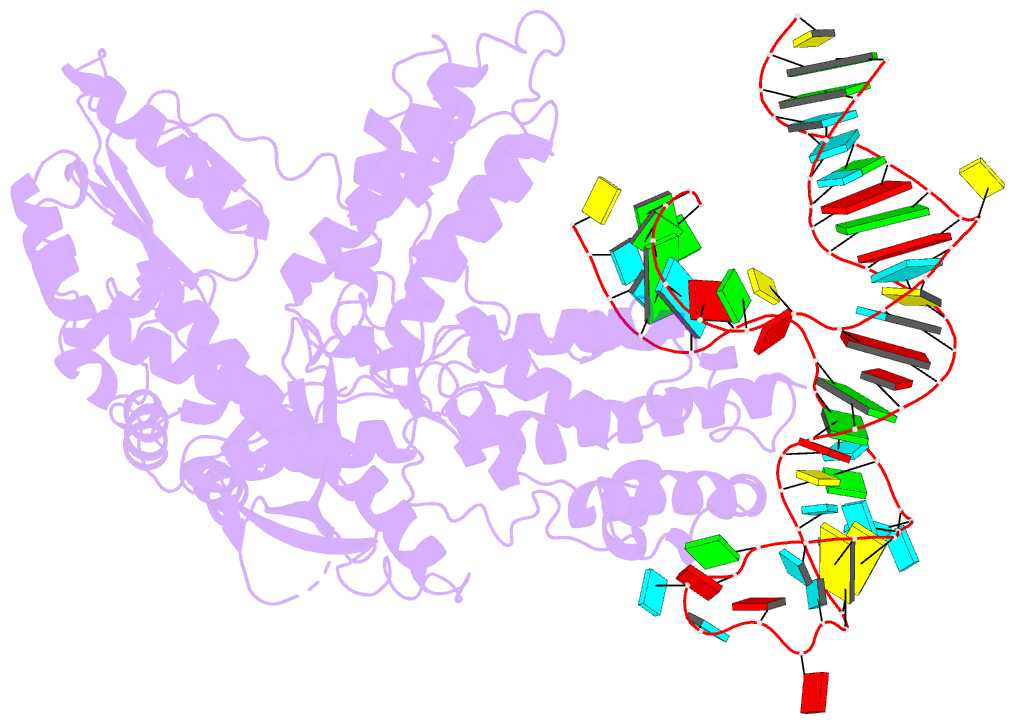
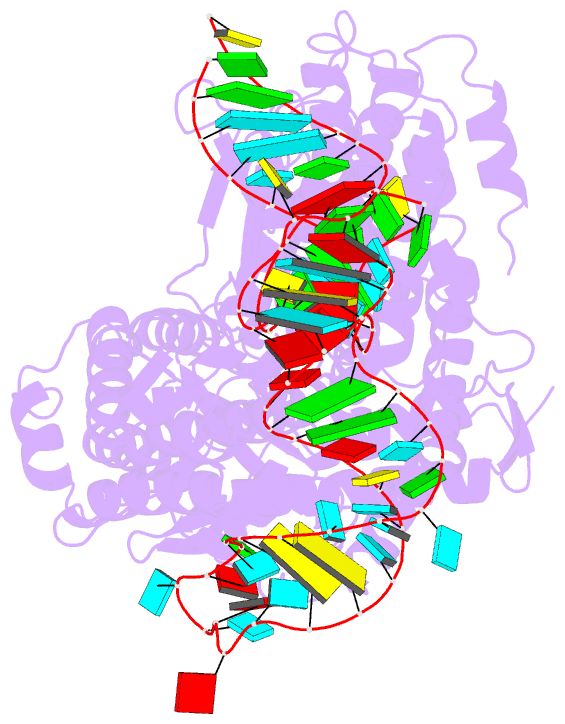
- Summary
- Structure of telomerase from candida tropicalis in
complexe with twj fragment of telomeric RNA
- Reference
-
Zhai LT, Rety S, Chen WF, Song ZY, Auguin D, Sun B, Dou
SX, Xi XG (2021): "Crystal
structures of N-terminally truncated telomerase reverse
transcriptase from fungi‡." Nucleic Acids
Res., 49, 4768-4781. doi: 10.1093/nar/gkab261.
- Abstract
- Telomerase plays critical roles in cellular aging, in
the emergence and/or development of cancer, and in the
capacity for stem-cell renewal, consists of a catalytic
telomerase reverse transcriptase (TERT) and a
template-encoding RNA (TER). TERs from diverse organisms
contain two conserved structural elements: the
template-pseudoknot (T-PK) and a helical three-way junction
(TWJ). Species-specific features of the structure and
function of telomerase make obtaining a more in-depth
understanding of the molecular mechanism of telomerase
particularly important. Here, we report the first
structural studies of N-terminally truncated TERTs from
Candida albicans and Candida tropicalis in apo form and
complexed with their respective TWJs in several
conformations. We found that Candida TERT proteins perform
only one round of telomere addition in the presence or
absence of PK/TWJ and display standard reverse
transcriptase activity. The C-terminal domain adopts at
least two extreme conformations and undergoes
conformational interconversion, which regulates the
catalytic activity. Most importantly, we identified a
conserved tertiary structural motif, called the U-motif,
which interacts with the reverse transcriptase domain and
is crucial for catalytic activity. Together these results
shed new light on the structure and mechanics of fungal
TERTs, which show common TERT characteristics, but also
display species-specific features.