Summary information and primary citation
- PDB-id
-
6ltr;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-RNA-DNA
- Method
- X-ray (2.51 Å)
- Summary
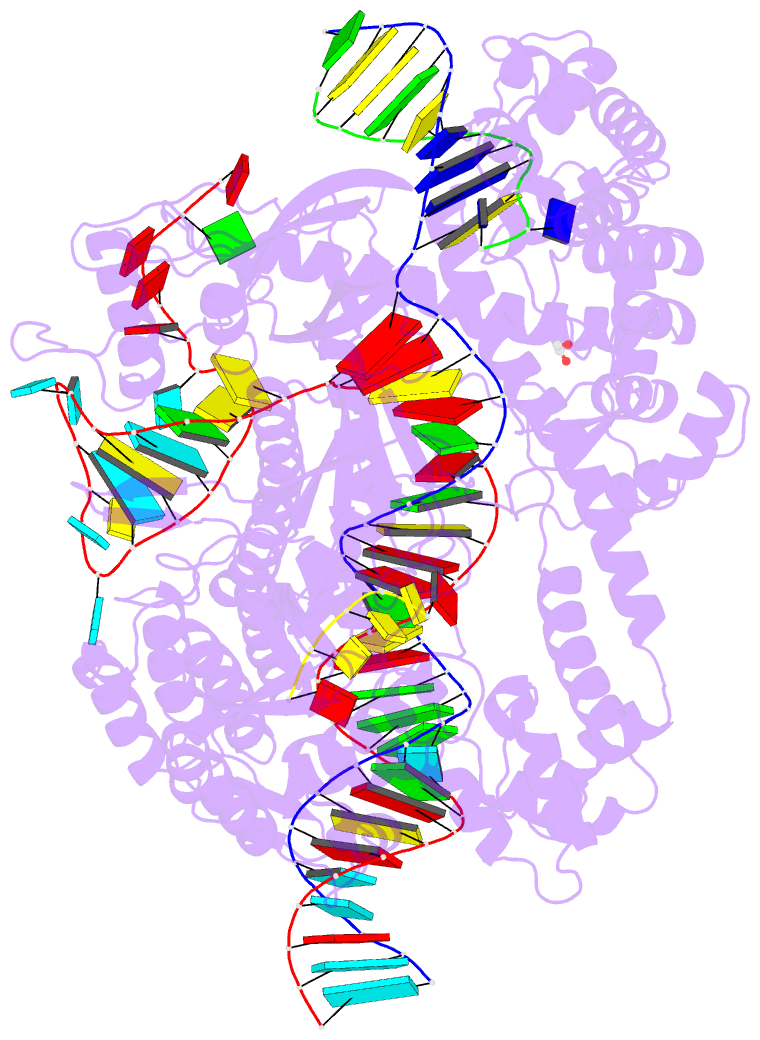
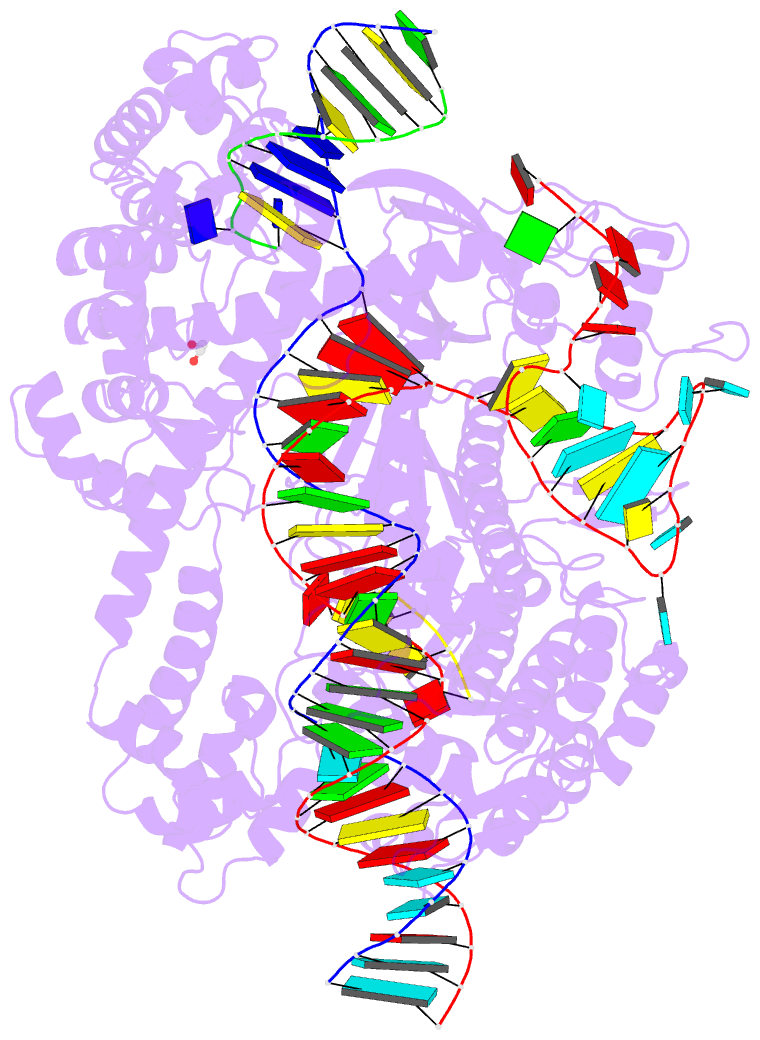
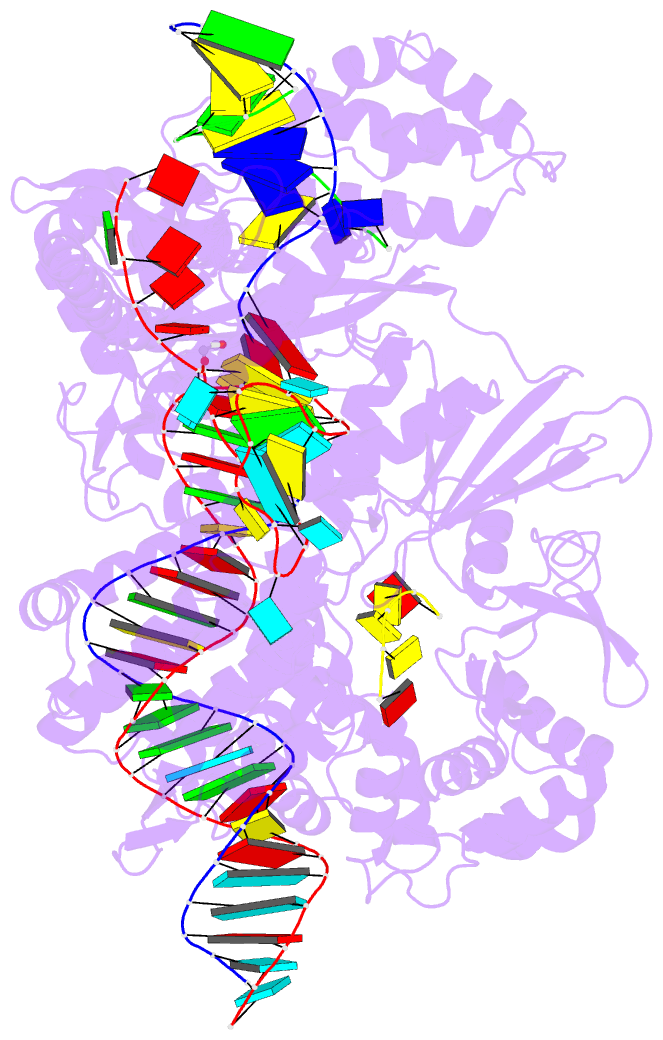
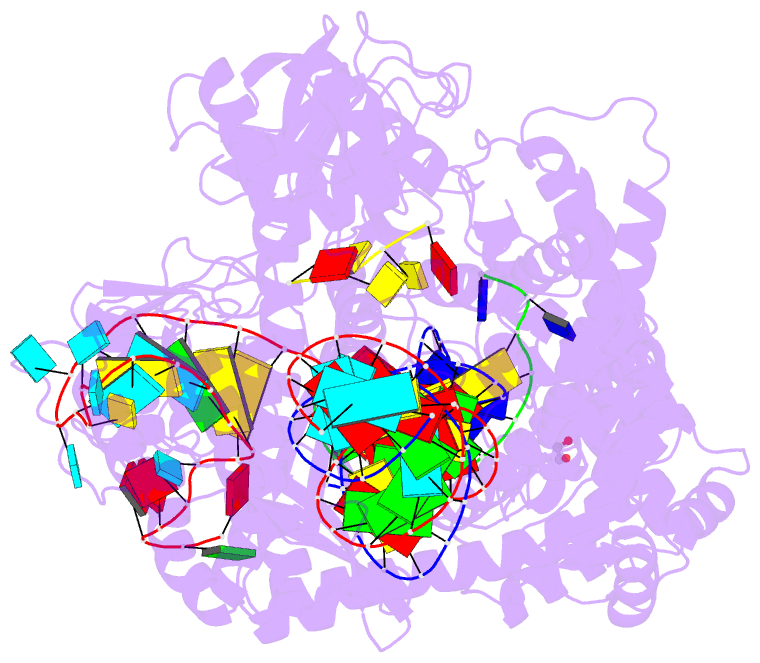
- Crystal structure of cas12i2 ternary complex with
single mg2+ bound in catalytic pocket
- Reference
-
Huang X, Sun W, Cheng Z, Chen M, Li X, Wang J, Sheng G,
Gong W, Wang Y (2020): "Structural
basis for two metal-ion catalysis of DNA cleavage by
Cas12i2." Nat Commun, 11,
5241. doi: 10.1038/s41467-020-19072-6.
- Abstract
- To understand how the RuvC catalytic domain of Class 2
Cas proteins cleaves DNA, it will be necessary to elucidate
the structures of RuvC-containing Cas complexes in their
catalytically competent states. Cas12i2 is a Class 2 type
V-I CRISPR-Cas endonuclease that cleaves target dsDNA by an
unknown mechanism. Here, we report structures of
Cas12i2-crRNA-DNA complexes and a Cas12i2-crRNA complex. We
reveal the mechanism of DNA recognition and cleavage by
Cas12i2, and activation of the RuvC catalytic pocket
induced by a conformational change of the Helical-II
domain. The seed region (nucleotides 1-8) is dispensable
for RuvC activation, but the duplex of the central spacer
(nucleotides 9-15) is required. We captured the catalytic
state of Cas12i2, with both metal ions and the ssDNA
substrate bound in the RuvC catalytic pocket. Together, our
studies provide significant insights into the DNA cleavage
mechanism by RuvC-containing Cas proteins.