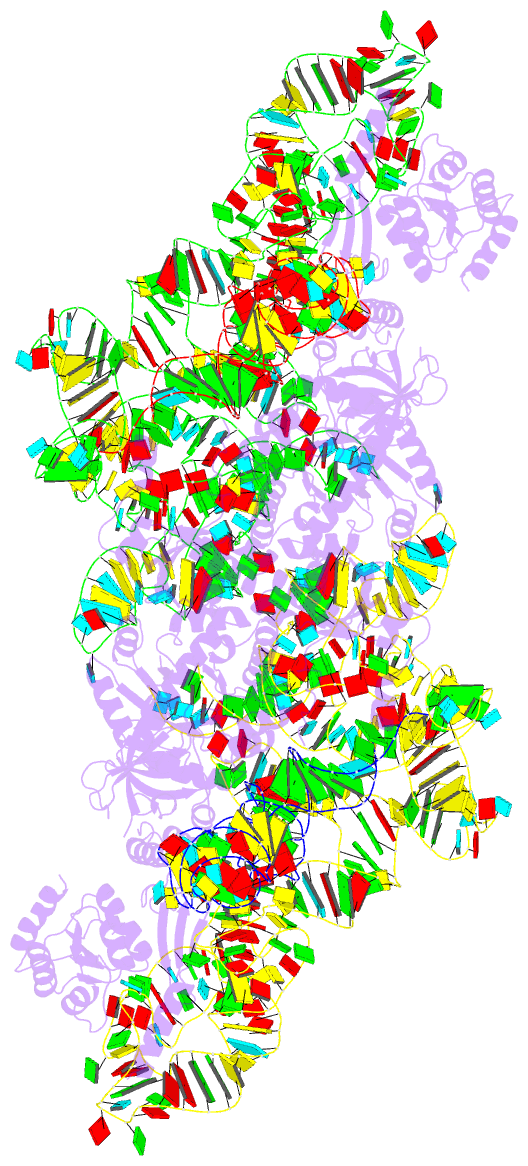
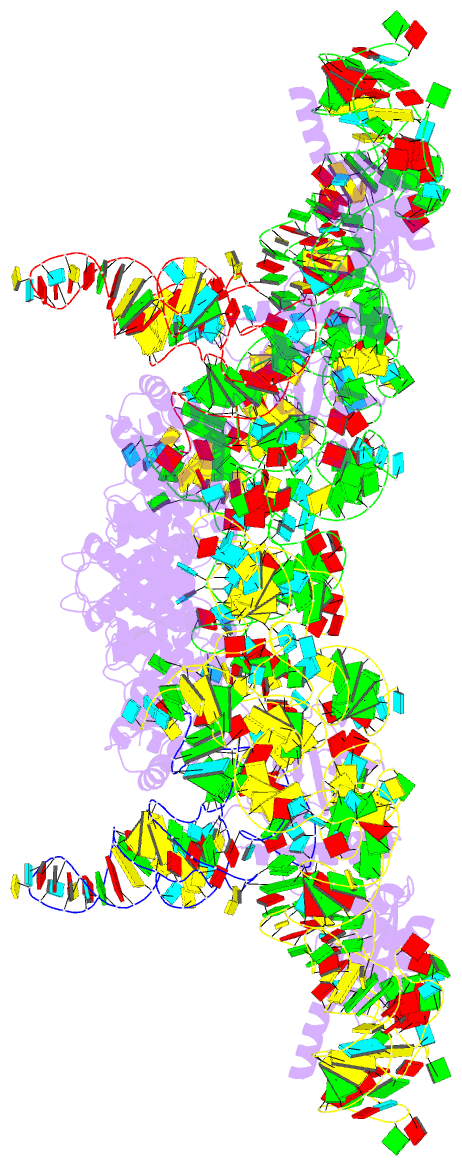
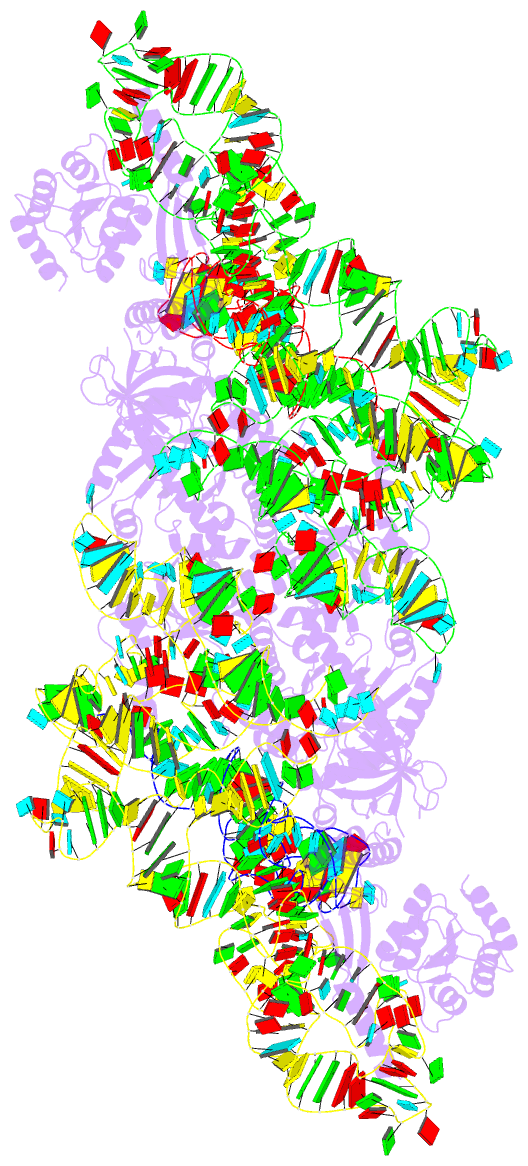
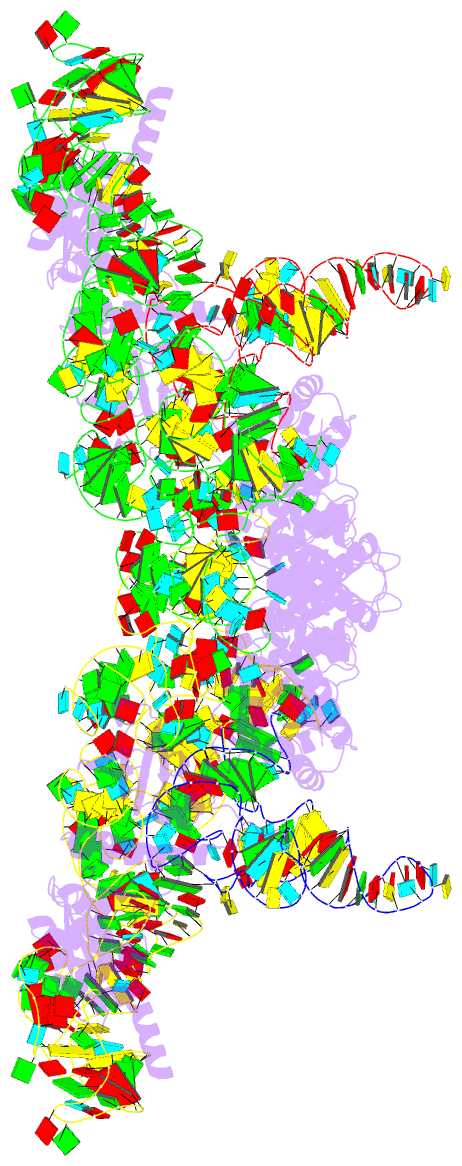
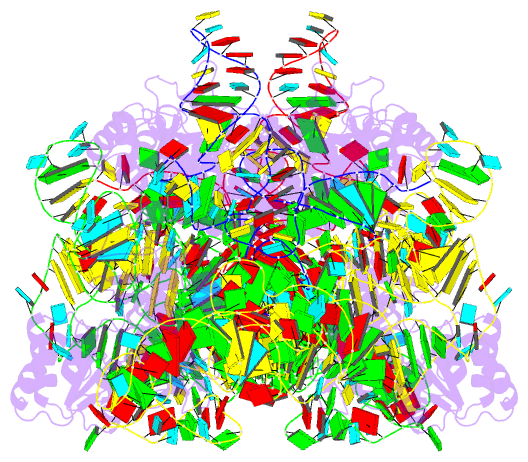
Summary information and primary citation
- PDB-id
-
6k0b;
SNAP-derived features in text and
JSON formats
- Class
- RNA binding protein-RNA
- Method
- cryo-EM (4.3 Å)
- Summary
- cryo-EM structure of archaeal ribonuclease p with
mature trna
- Reference
-
Wan F, Wang Q, Tan J, Tan M, Chen J, Shi S, Lan P, Wu J,
Lei M (2019): "Cryo-electron
microscopy structure of an archaeal ribonuclease P
holoenzyme." Nat Commun,
10, 2617. doi: 10.1038/s41467-019-10496-3.
- Abstract
- Ribonuclease P (RNase P) is an essential ribozyme
responsible for tRNA 5' maturation. Here we report the
cryo-EM structures of Methanocaldococcus jannaschii (Mja)
RNase P holoenzyme alone and in complex with a tRNA
substrate at resolutions of 4.6 Å and 4.3 Å, respectively.
The structures reveal that the subunits of MjaRNase P are
strung together to organize the holoenzyme in a dimeric
conformation required for efficient catalysis. The
structures also show that archaeal RNase P is a functional
chimera of bacterial and eukaryal RNase Ps that possesses
bacterial-like two RNA-based anchors and a eukaryal-like
protein-aided stabilization mechanism. The 3'-RCCA sequence
of tRNA, which is a key recognition element for bacterial
RNase P, is dispensable for tRNA recognition by MjaRNase P.
The overall organization of MjaRNase P, particularly within
the active site, is similar to those of bacterial and
eukaryal RNase Ps, suggesting a universal catalytic
mechanism for all RNase Ps.