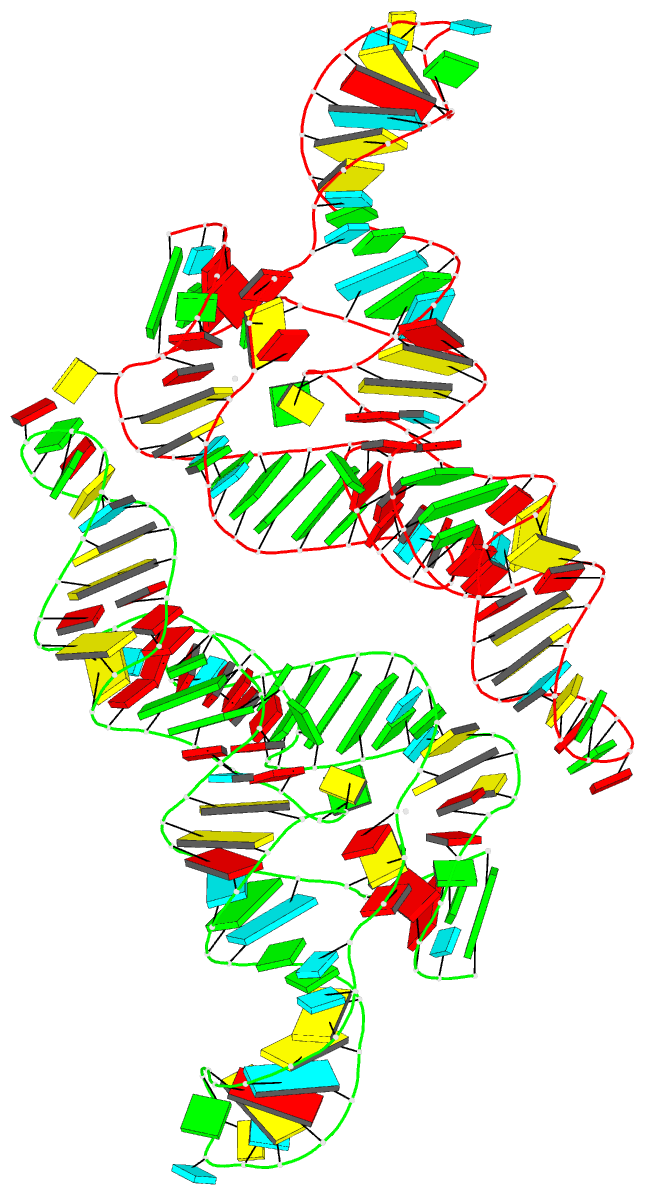
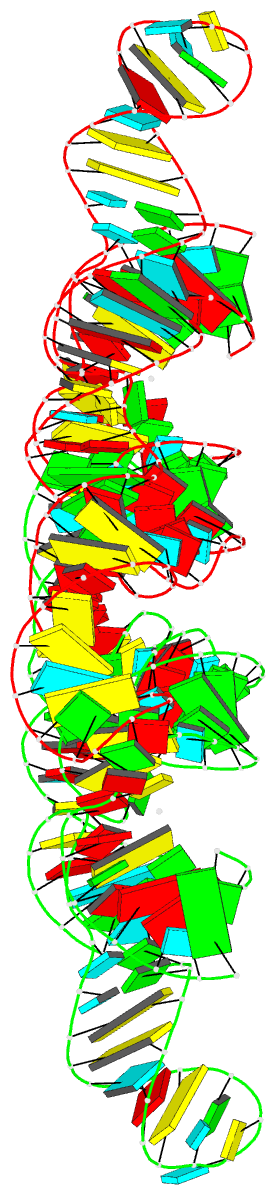
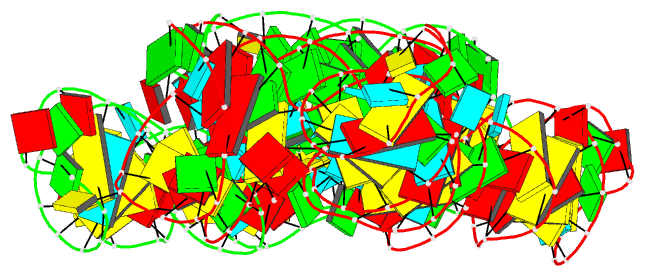
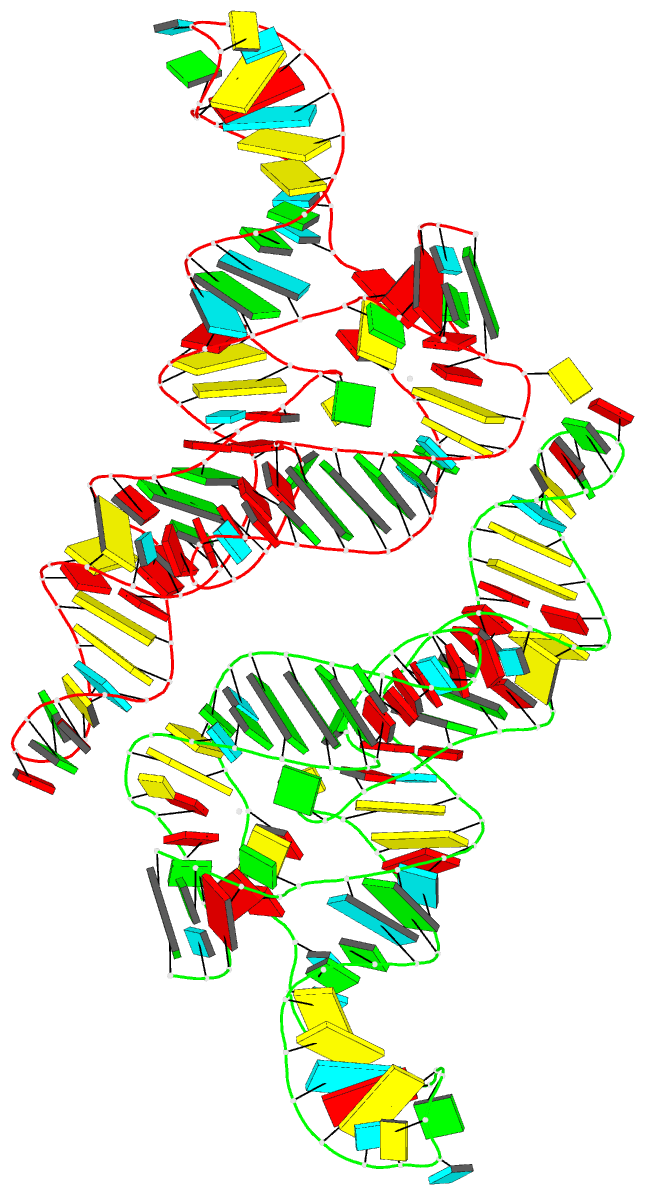
Summary information and primary citation
- PDB-id
-
6dmc;
SNAP-derived features in text and
JSON formats
- Class
- RNA
- Method
- X-ray (2.2 Å)
- Summary
- Ppgpp riboswitch bound to ppgpp, native structure
- Reference
-
Peselis A, Serganov A (2018): "ykkC
riboswitches employ an add-on helix to adjust specificity
for polyanionic ligands." Nat. Chem. Biol.,
14, 887-894. doi: 10.1038/s41589-018-0114-4.
- Abstract
- The ykkC family of bacterial riboswitches combines
several widespread classes that have similar secondary
structures and consensus motifs but control different genes
in response to different cellular metabolites. Here we
report the crystal structures of two distinct ykkC
riboswitches specifically bound to their cognate ligand
ppGpp, a second messenger involved in stress response, or
PRPP, a precursor in purine biosynthesis. Both RNAs adopt
similar structures and contain a conserved core previously
observed in the guanidine-specific ykkC riboswitch.
However, ppGpp and PRPP riboswitches uniquely employ an
additional helical element that joins the ends of the
ligand-sensing domains and creates a tunnel for direct and
Mg<sub>2+</sub>-mediated binding of ligands.
Mutational and footprinting experiments highlight the
importance of conserved nucleotides forming the tunnel and
long-distance contacts for ligand binding and genetic
response. Our work provides new insights into the
specificity of riboswitches and gives a unique opportunity
for future studies of RNA evolution.