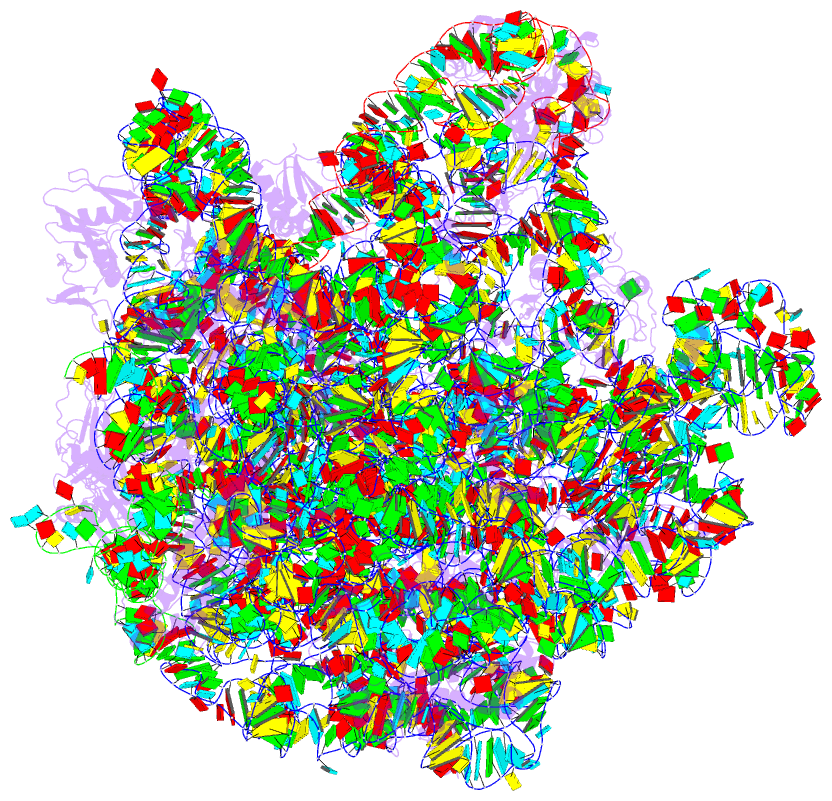
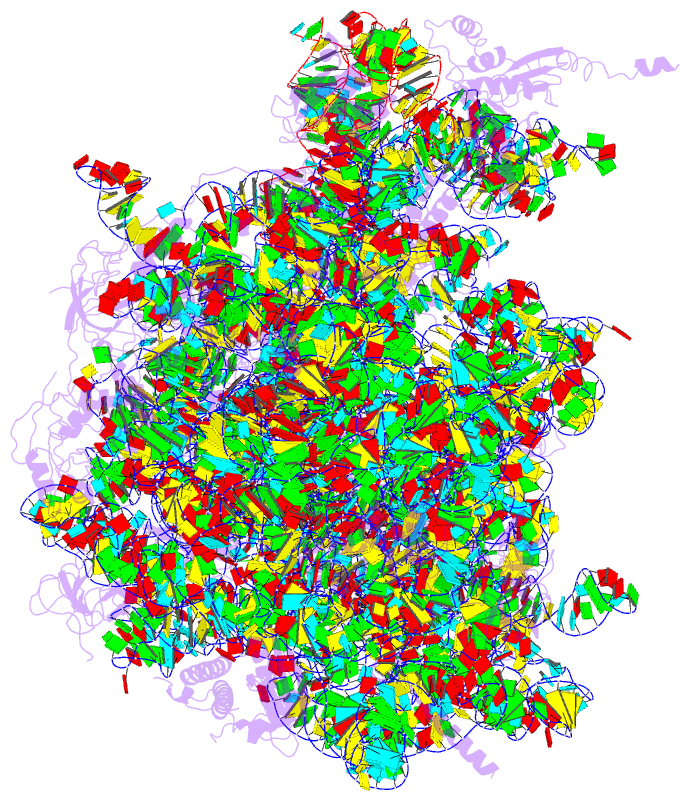
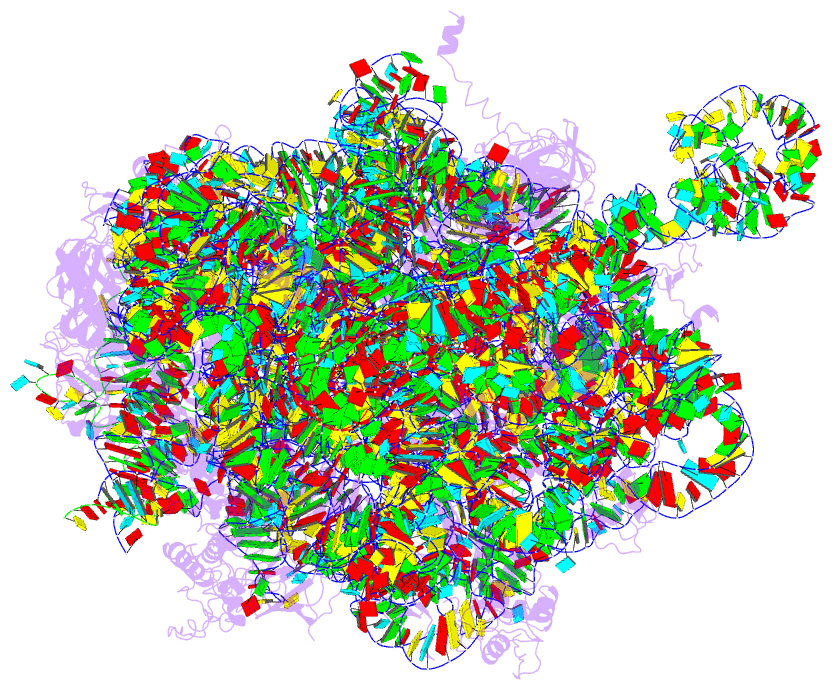
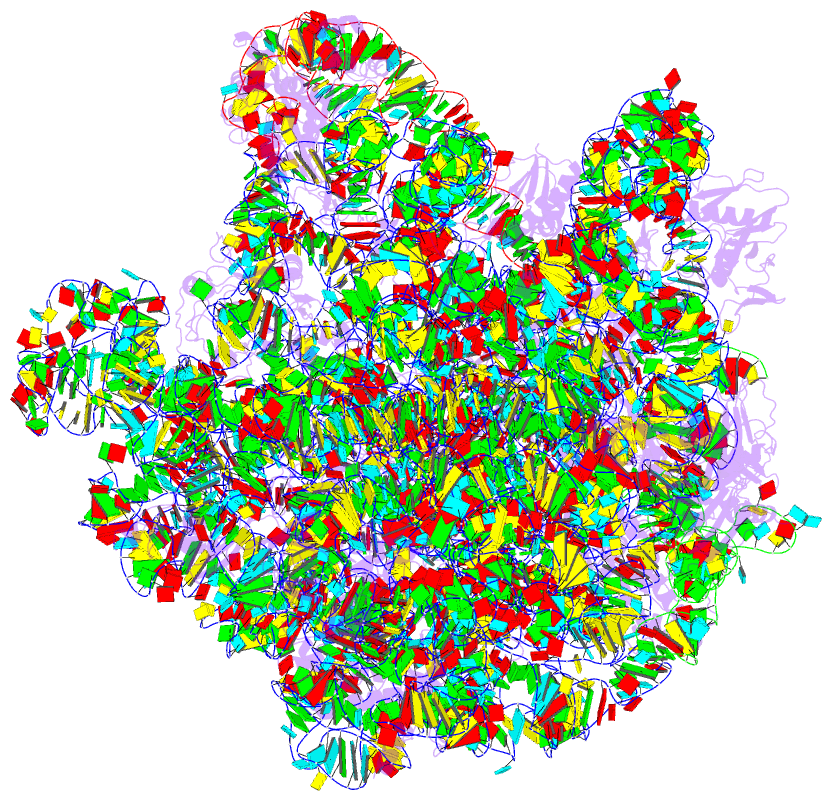
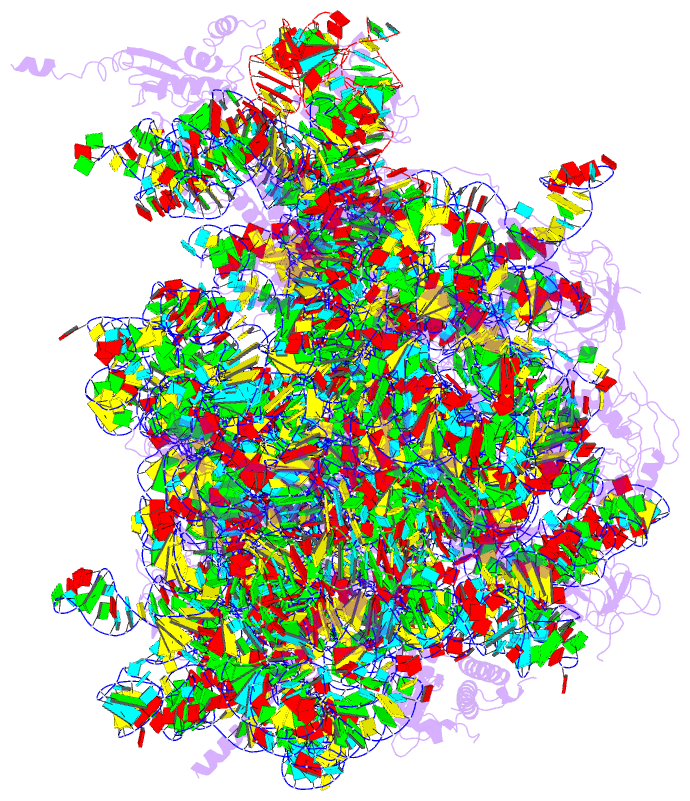
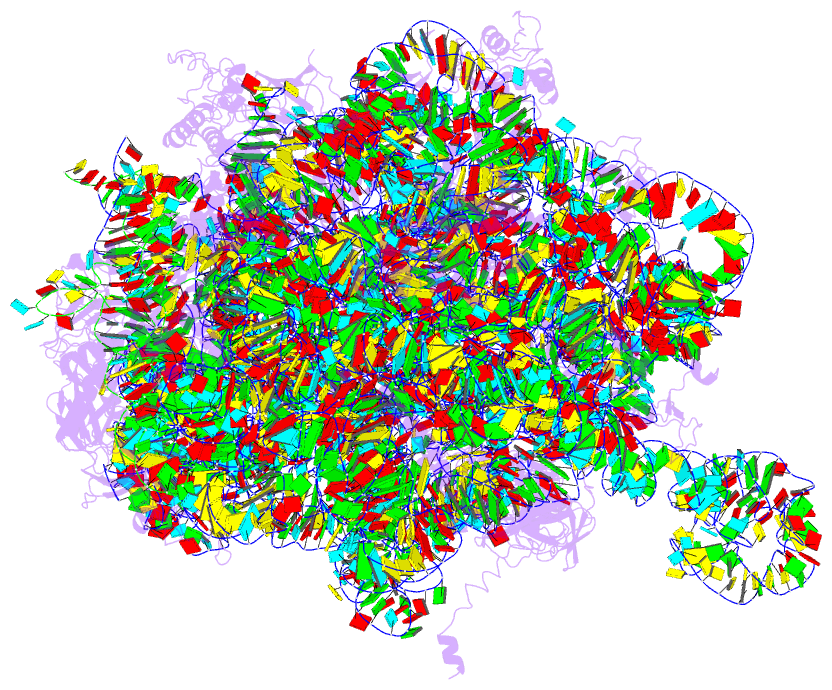
Summary information and primary citation
- PDB-id
-
5x8t;
SNAP-derived features in text and
JSON formats
- Class
- ribosome
- Method
- cryo-EM (3.3 Å)
- Summary
- Structure of the 50s large subunit of chloroplast
ribosome from spinach
- Reference
-
Ahmed T, Shi J, Bhushan S (2017): "Unique
localization of the plastid-specific ribosomal proteins
in the chloroplast ribosome small subunit provides
mechanistic insights into the chloroplastic
translation." Nucleic Acids Res.,
45, 8581-8595. doi: 10.1093/nar/gkx499.
- Abstract
- Chloroplastic translation is mediated by a
bacterial-type 70S chloroplast ribosome. During the
evolution, chloroplast ribosomes have acquired five
plastid-specific ribosomal proteins or PSRPs (cS22, cS23,
bTHXc, cL37 and cL38) which have been suggested to play
important regulatory roles in translation. However, their
exact locations on the chloroplast ribosome remain elusive
due to lack of a high-resolution structure, hindering our
progress to understand their possible roles. Here we
present a cryo-EM structure of the 70S chloroplast ribosome
from spinach resolved to 3.4 Å and focus our discussion
mainly on the architecture of the 30S small subunit (SSU)
which is resolved to 3.7 Å. cS22 localizes at the SSU foot
where it seems to compensate for the deletions in 16S rRNA.
The mRNA exit site is highly remodeled due to the presence
of cS23 suggesting an alternative mode of translation
initiation. bTHXc is positioned at the SSU head and appears
to stabilize the intersubunit bridge B1b during thermal
fluctuations. The translation factor plastid pY binds to
the SSU on the intersubunit side and interacts with the
conserved nucleotide bases involved in decoding. Most of
the intersubunit bridges are conserved compared to the
bacteria, except for a new bridge involving uL2c and
bS6c.