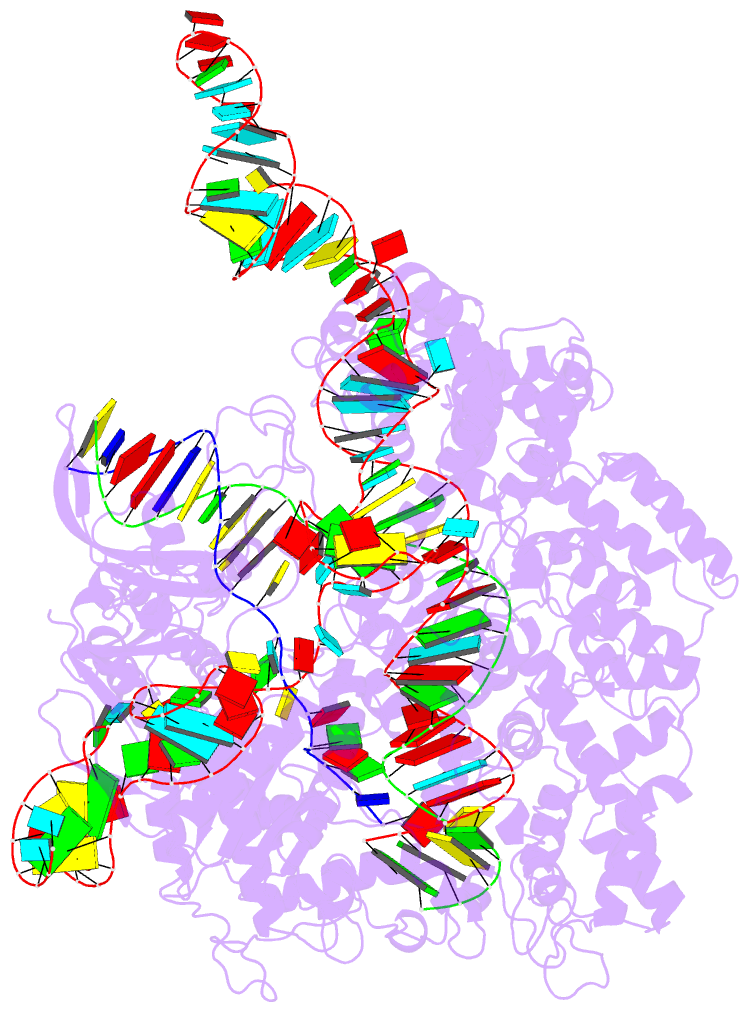
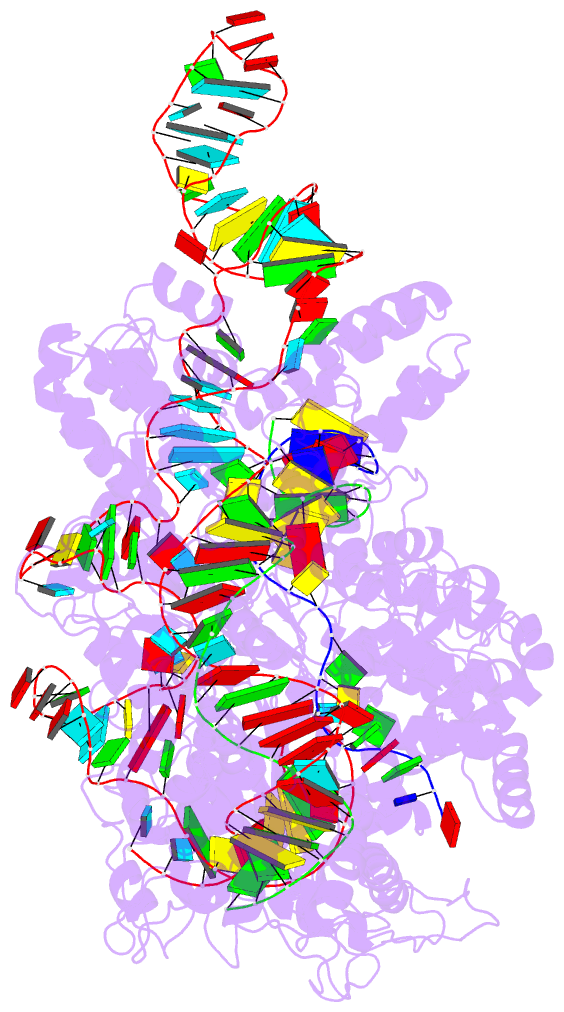
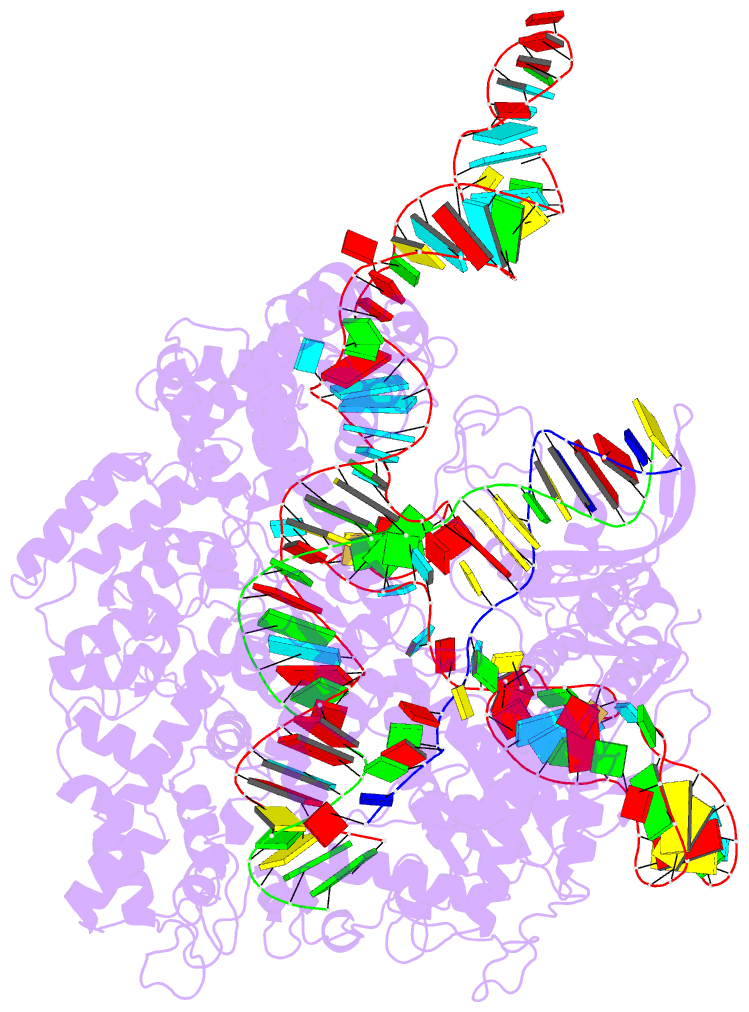
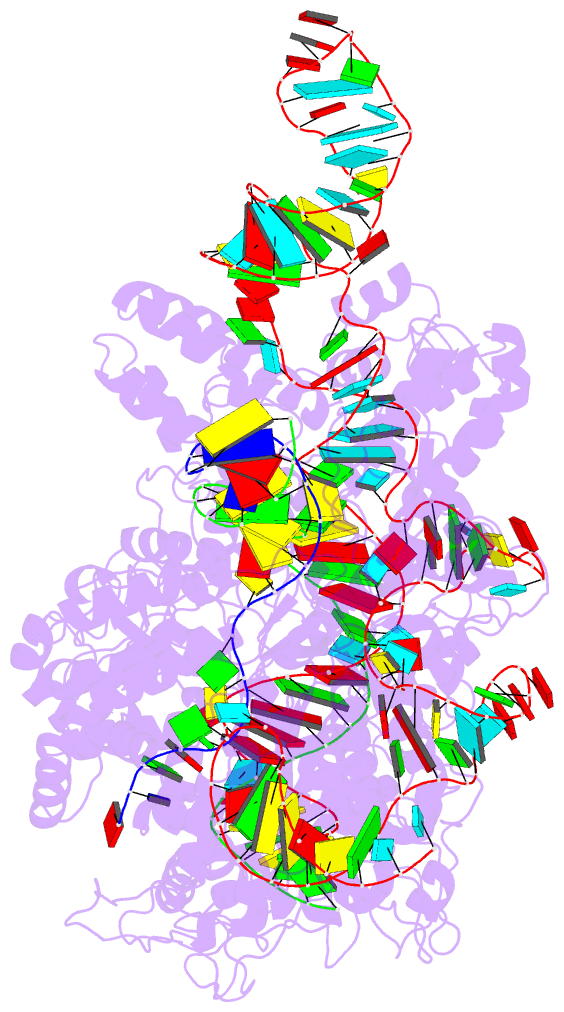
Summary information and primary citation
- PDB-id
-
5f9r;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-DNA-RNA
- Method
- X-ray (3.4 Å)
- Summary
- Crystal structure of catalytically-active streptococcus
pyogenes crispr-cas9 in complex with single-guided RNA and
double-stranded DNA primed for target DNA cleavage
- Reference
-
Jiang F, Taylor DW, Chen JS, Kornfeld JE, Zhou K,
Thompson AJ, Nogales E, Doudna JA (2016): "Structures
of a CRISPR-Cas9 R-loop complex primed for DNA
cleavage." Science, 351,
867-871. doi: 10.1126/science.aad8282.
- Abstract
- Bacterial adaptive immunity and genome engineering
involving the CRISPR (clustered regularly interspaced short
palindromic repeats)-associated (Cas) protein Cas9 begin
with RNA-guided DNA unwinding to form an RNA-DNA hybrid and
a displaced DNA strand inside the protein. The role of this
R-loop structure in positioning each DNA strand for
cleavage by the two Cas9 nuclease domains is unknown. We
determine molecular structures of the catalytically active
Streptococcus pyogenes Cas9 R-loop that show the displaced
DNA strand located near the RuvC nuclease domain active
site. These protein-DNA interactions, in turn, position the
HNH nuclease domain adjacent to the target DNA strand
cleavage site in a conformation essential for concerted DNA
cutting. Cas9 bends the DNA helix by 30°, providing the
structural distortion needed for R-loop formation.