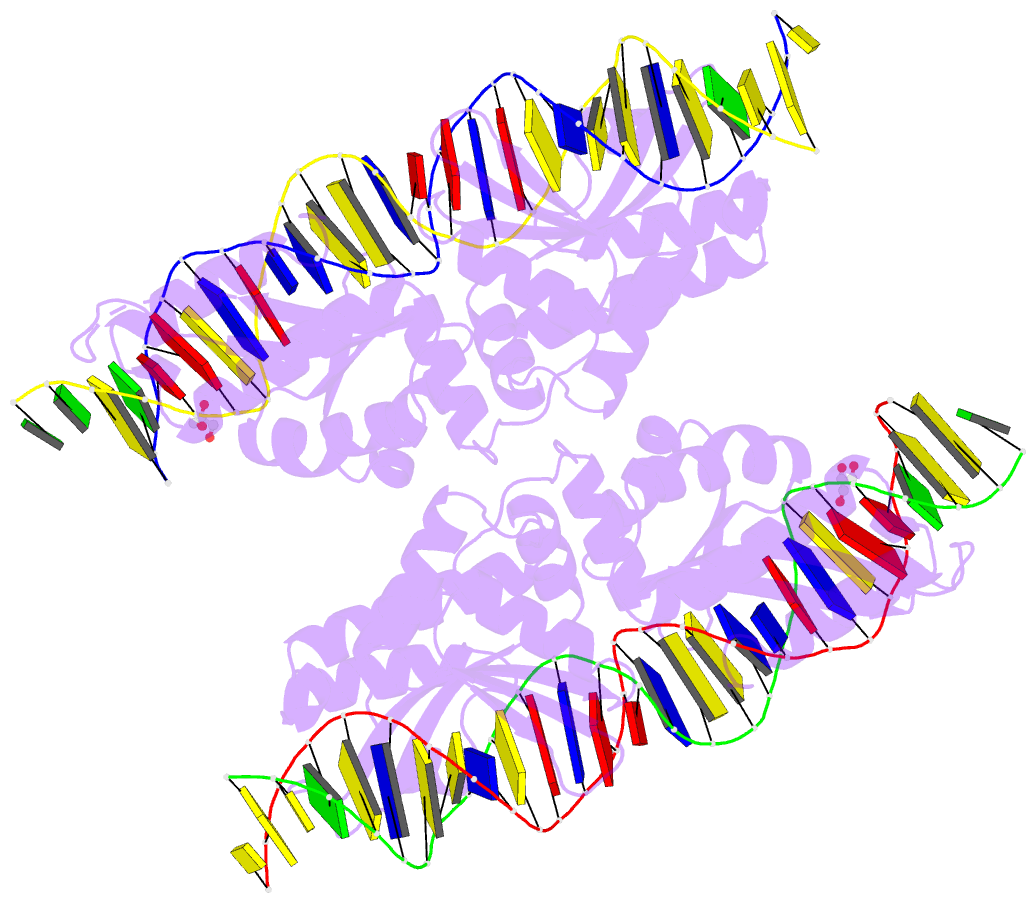
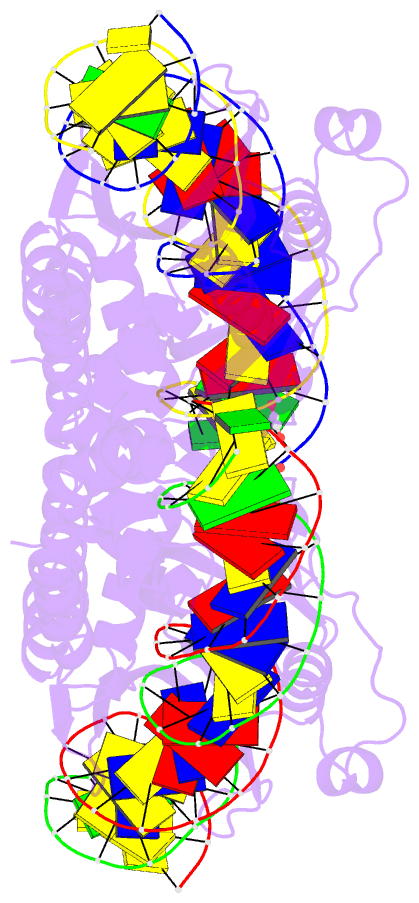
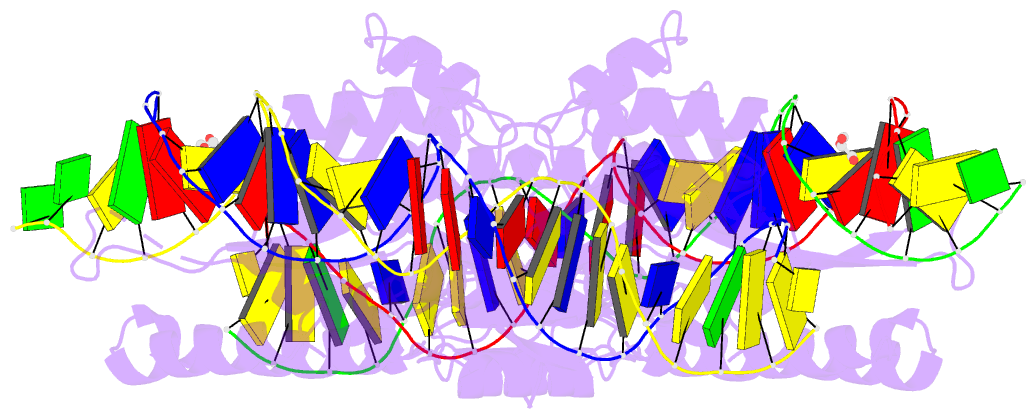
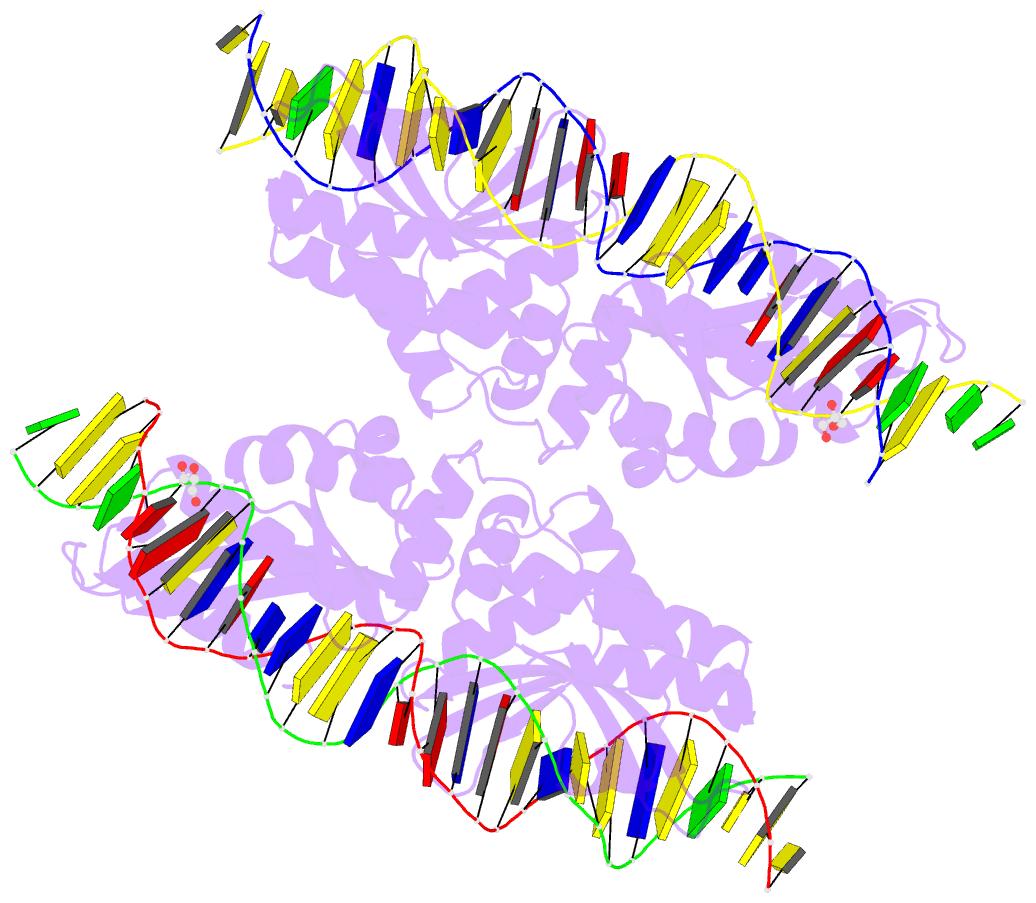
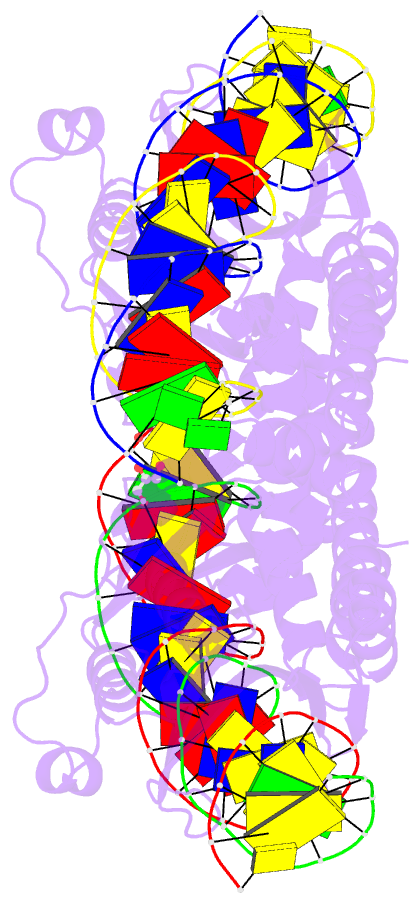
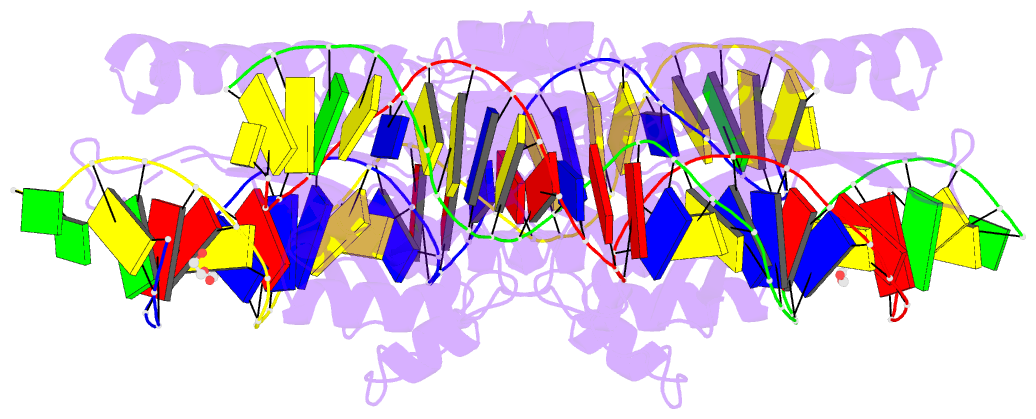
Summary information and primary citation
- PDB-id
-
5esp;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-DNA
- Method
- X-ray (2.995 Å)
- Summary
- Crystal structure of laglidadg meganuclease i-panmi
with coordinated calcium ions
- Reference
-
Lambert AR, Hallinan JP, Shen BW, Chik JK, Bolduc JM,
Kulshina N, Robins LI, Kaiser BK, Jarjour J, Havens K,
Scharenberg AM, Stoddard BL (2016): "Indirect
DNA Sequence Recognition and Its Impact on Nuclease
Cleavage Activity." Structure,
24, 862-873. doi: 10.1016/j.str.2016.03.024.
- Abstract
- LAGLIDADG meganucleases are DNA cleaving enzymes used
for genome engineering. While their cleavage specificity
can be altered using several protein engineering and
selection strategies, their overall targetability is
limited by highly specific indirect recognition of the
central four base pairs within their recognition sites. In
order to examine the physical basis of indirect sequence
recognition and to expand the number of such nucleases
available for genome engineering, we have determined the
target sites, DNA-bound structures, and central four
cleavage fidelities of nine related enzymes. Subsequent
crystallographic analyses of a meganuclease bound to two
noncleavable target sites, each containing a single
inactivating base pair substitution at its center,
indicates that a localized slip of the mutated base pair
causes a small change in the DNA backbone conformation that
results in a loss of metal occupancy at one binding site,
eliminating cleavage activity.