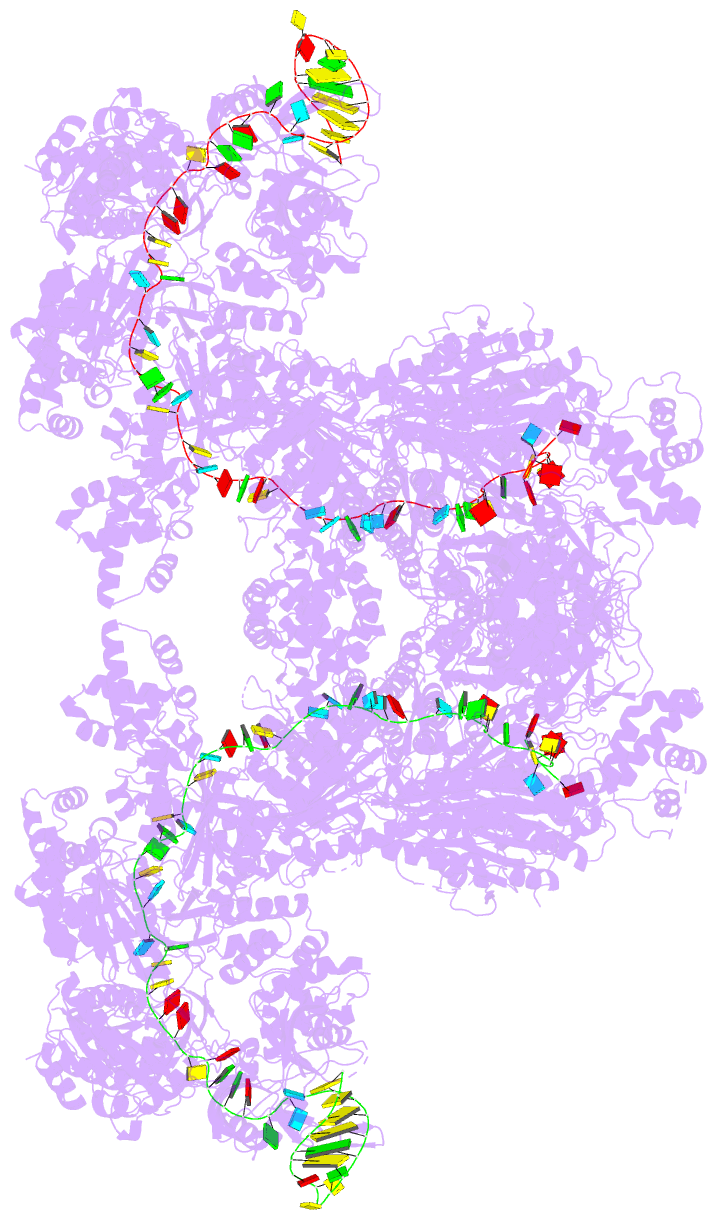
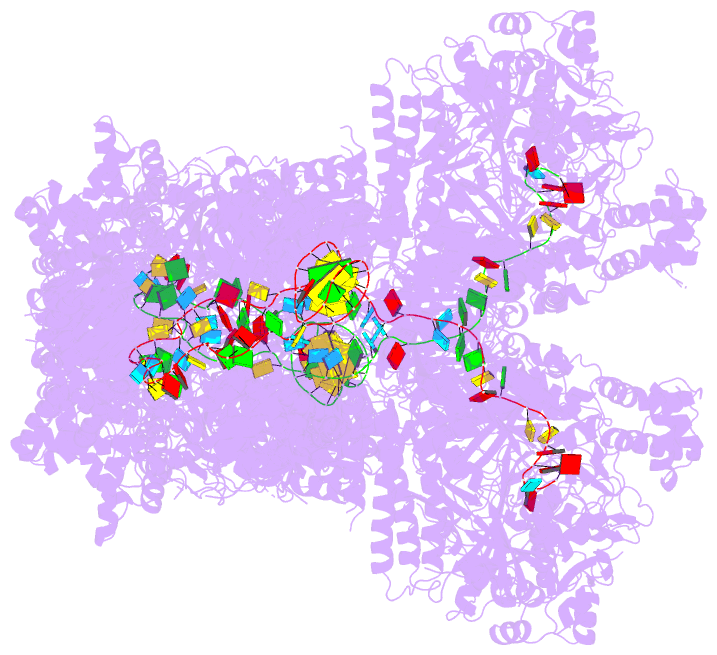
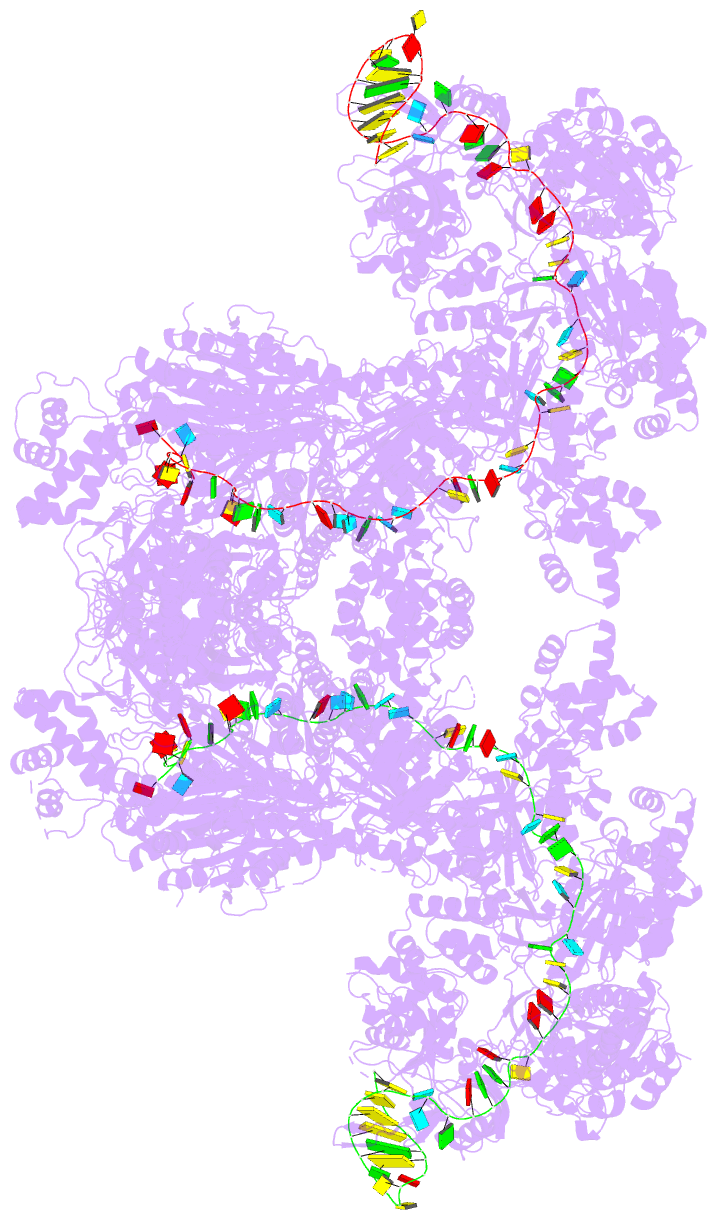
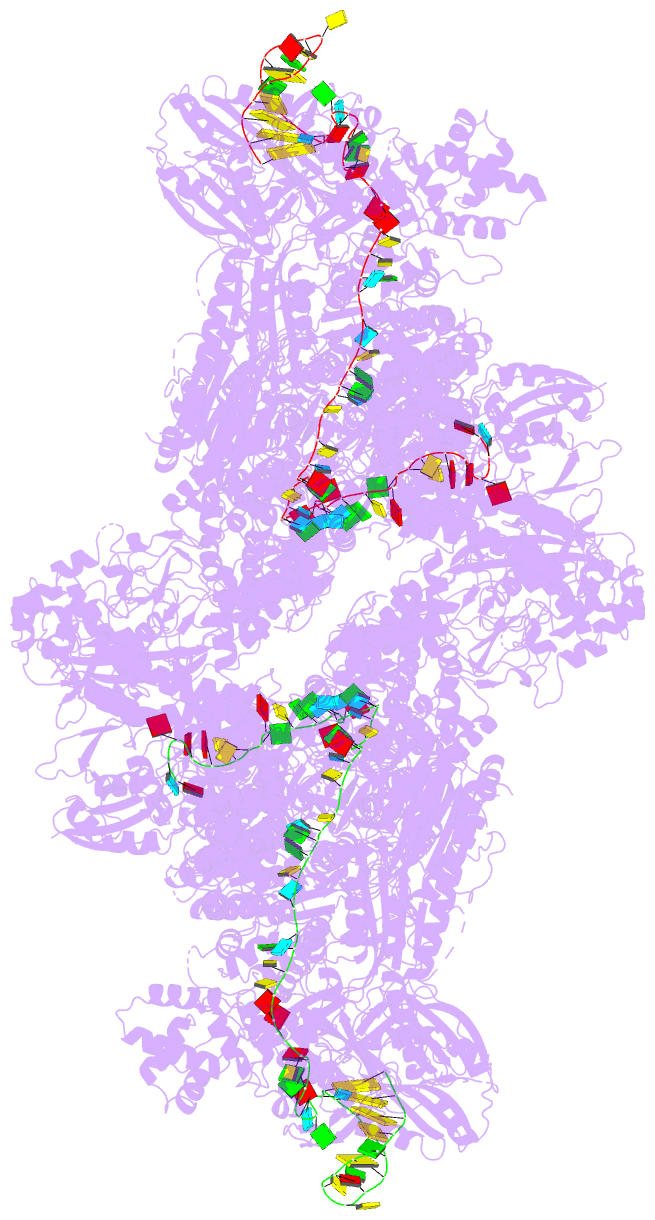
Summary information and primary citation
- PDB-id
-
5cd4;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-RNA
- Method
- X-ray (3.2 Å)
- Summary
- The type ie crispr cascade complex from e. coli, with
two assemblies in the asymmetric unit arranged
back-to-back
- Reference
-
van Erp PB, Jackson RN, Carter J, Golden SM, Bailey S,
Wiedenheft B (2015): "Mechanism
of CRISPR-RNA guided recognition of DNA targets in
Escherichia coli." Nucleic Acids Res.,
43, 8381-8391. doi: 10.1093/nar/gkv793.
- Abstract
- In bacteria and archaea, short fragments of foreign DNA
are integrated into Clustered Regularly Interspaced Short
Palindromic Repeat (CRISPR) loci, providing a molecular
memory of previous encounters with foreign genetic
elements. In Escherichia coli, short CRISPR-derived RNAs
are incorporated into a multi-subunit surveillance complex
called Cascade (CRISPR-associated complex for antiviral
defense). Recent structures of Cascade capture snapshots of
this seahorse-shaped RNA-guided surveillance complex before
and after binding to a DNA target. Here we determine a 3.2
Å x-ray crystal structure of Cascade in a new crystal form
that provides insight into the mechanism of double-stranded
DNA binding. Molecular dynamic simulations performed using
available structures reveal functional roles for residues
in the tail, backbone and belly subunits of Cascade that
are critical for binding double-stranded DNA. Structural
comparisons are used to make functional predictions and
these predictions are tested in vivo and in vitro.
Collectively, the results in this study reveal underlying
mechanisms involved in target-induced conformational
changes and highlight residues important in DNA binding and
protospacer adjacent motif recognition.