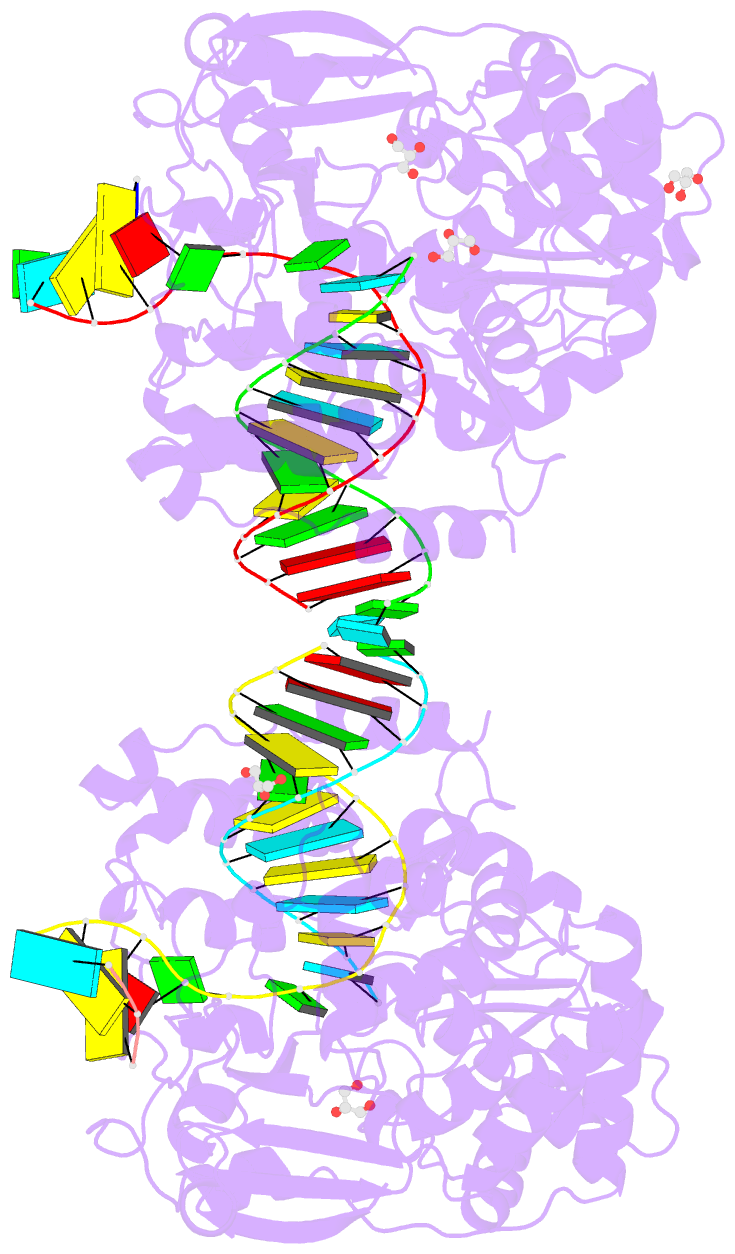
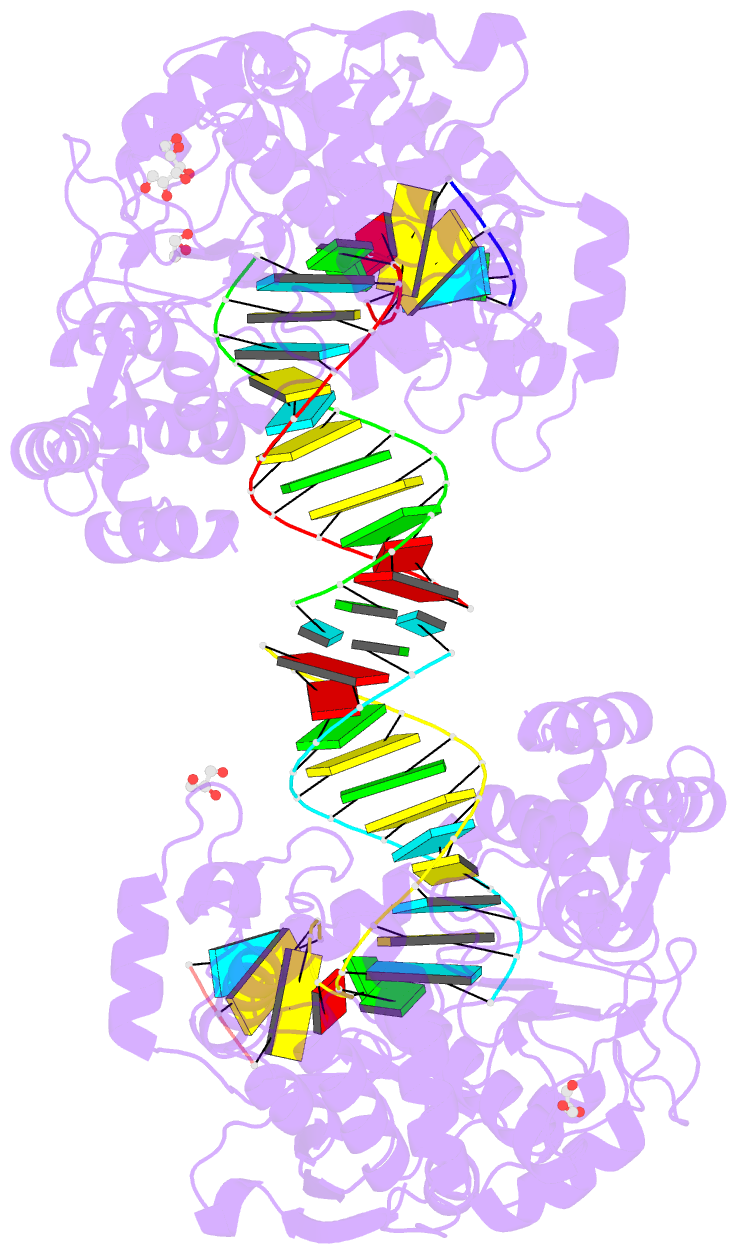
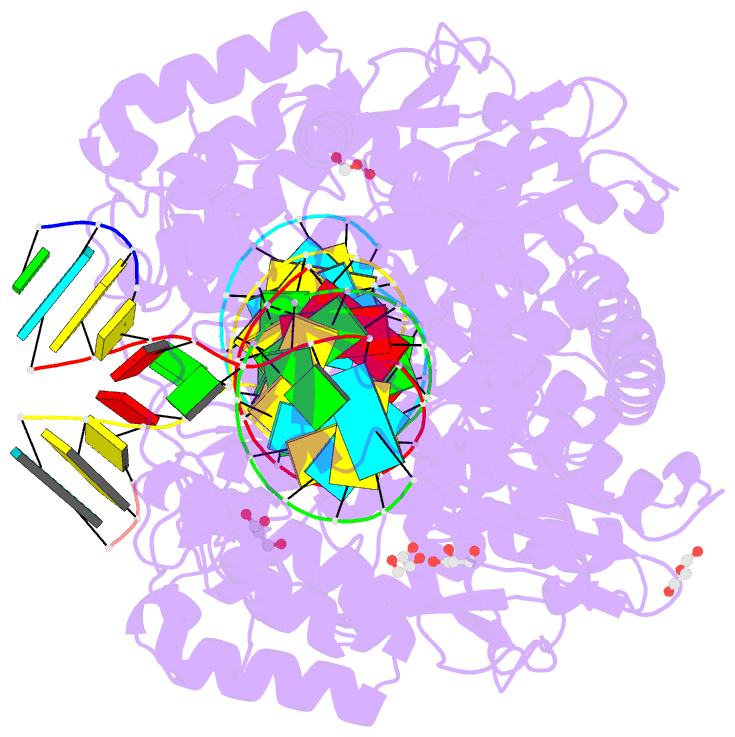
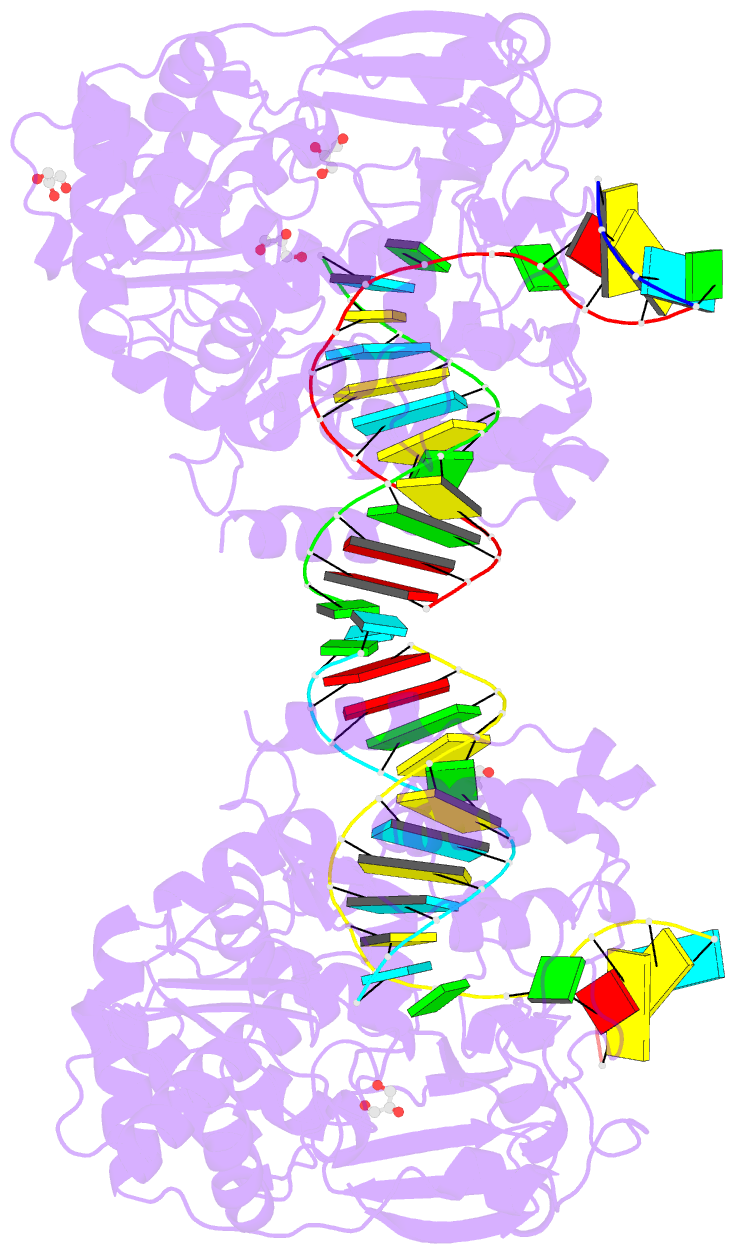
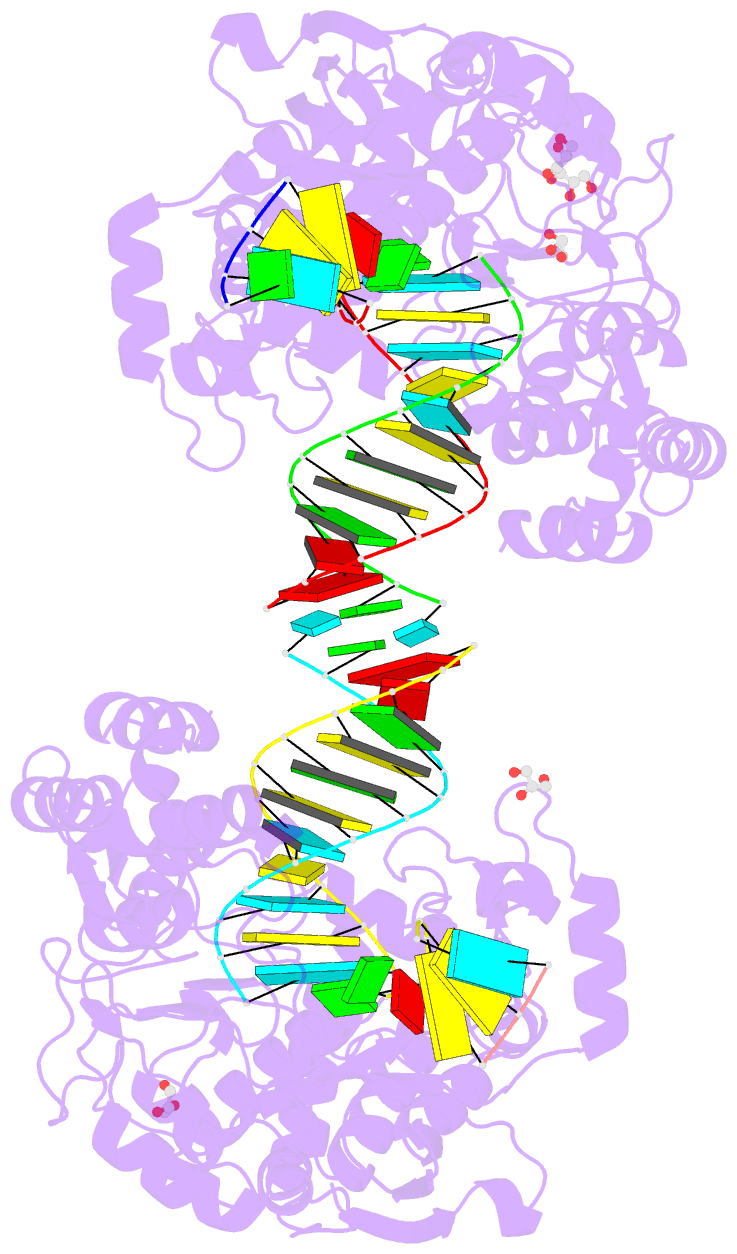
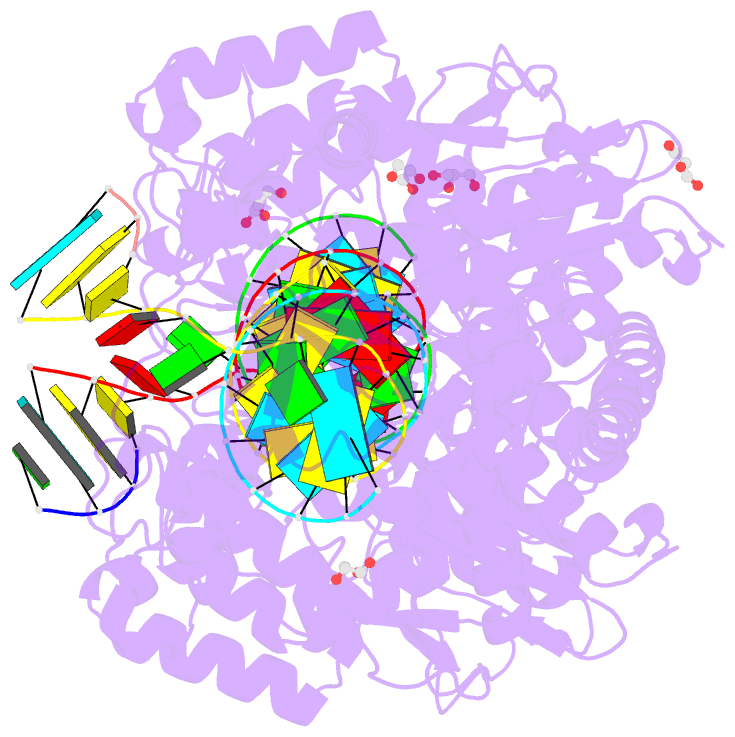
Summary information and primary citation
- PDB-id
-
4k4s;
SNAP-derived features in text and
JSON formats
- Class
- transferase-RNA
- Method
- X-ray (2.4 Å)
- Summary
- Poliovirus polymerase elongation complex (r3_form)
- Reference
-
Gong P, Kortus MG, Nix JC, Davis RE, Peersen OB (2013):
"Structures
of coxsackievirus, rhinovirus, and poliovirus polymerase
elongation complexes solved by engineering RNA mediated
crystal contacts." Plos One,
8, e60272. doi: 10.1371/journal.pone.0060272.
- Abstract
- RNA-dependent RNA polymerases play a vital role in the
growth of RNA viruses where they are responsible for genome
replication, but do so with rather low fidelity that allows
for the rapid adaptation to different host cell
environments. These polymerases are also a target for
antiviral drug development. However, both drug discovery
efforts and our understanding of fidelity determinants have
been hampered by a lack of detailed structural information
about functional polymerase-RNA complexes and the
structural changes that take place during the elongation
cycle. Many of the molecular details associated with
nucleotide selection and catalysis were revealed in our
recent structure of the poliovirus polymerase-RNA complex
solved by first purifying and then crystallizing stalled
elongation complexes. In the work presented here we extend
that basic methodology to determine nine new structures of
poliovirus, coxsackievirus, and rhinovirus elongation
complexes at 2.2-2.9 Å resolution. The structures highlight
conserved features of picornaviral polymerases and the
interactions they make with the template and product RNA
strands, including a tight grip on eight basepairs of the
nascent duplex, a fully pre-positioned templating
nucleotide, and a conserved binding pocket for the +2
position template strand base. At the active site we see a
pre-bound magnesium ion and there is conservation of a
non-standard backbone conformation of the template strand
in an interaction that may aid in triggering RNA
translocation via contact with the conserved polymerase
motif B. Moreover, by engineering plasticity into RNA-RNA
contacts, we obtain crystal forms that are capable of
multiple rounds of in-crystal catalysis and RNA
translocation. Together, the data demonstrate that
engineering flexible RNA contacts to promote crystal
lattice formation is a versatile platform that can be used
to solve the structures of viral RdRP elongation complexes
and their catalytic cycle intermediates.