Summary information and primary citation
- PDB-id
-
3vxv;
SNAP-derived features in text and
JSON formats
- Class
- hydrolase-DNA
- Method
- X-ray (2.0 Å)
- Summary
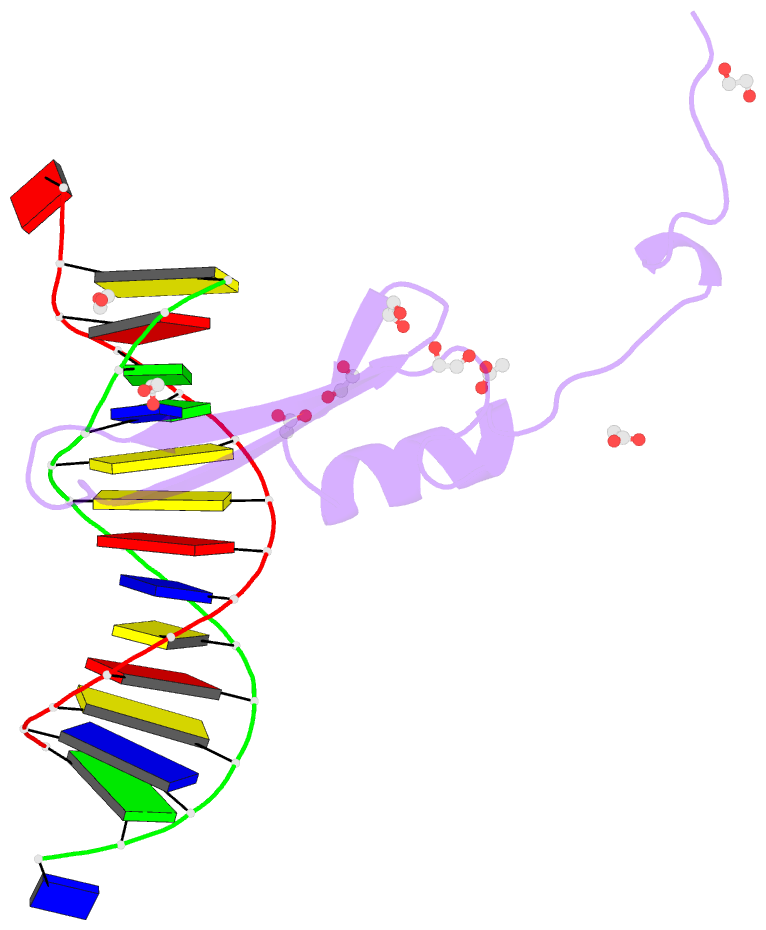
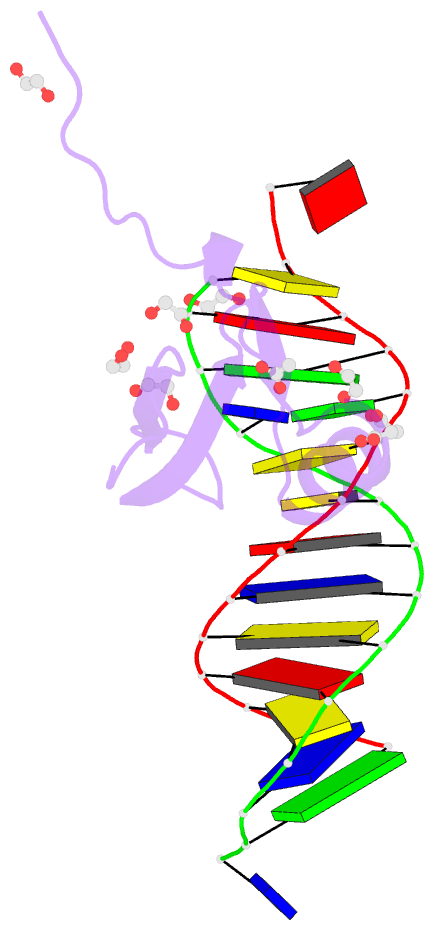
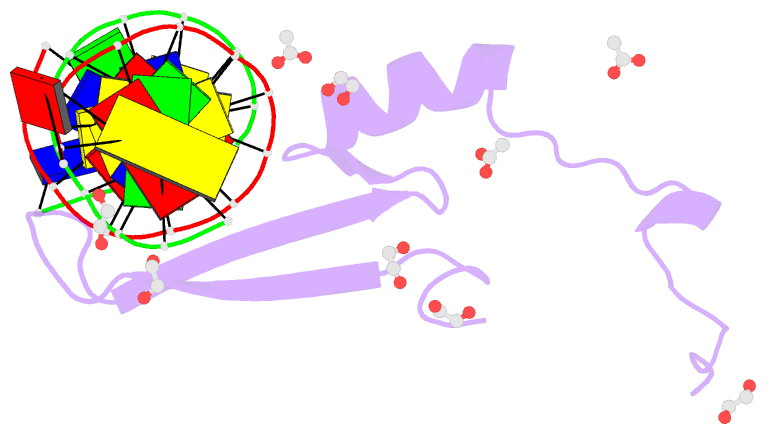
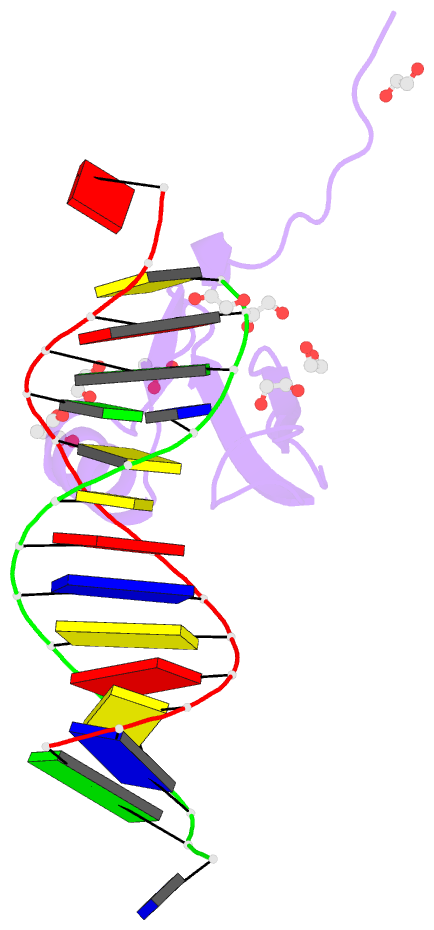
- Crystal structure of methyl cpg binding domain of mbd4
in complex with the 5mcg-tg sequence
- Reference
-
Otani J, Arita K, Kato T, Kinoshita M, Kimura H, Suetake
I, Tajima S, Ariyoshi M, Shirakawa M (2013): "Structural
basis of the versatile DNA recognition ability of the
methyl-CpG binding domain of methyl-CpG binding domain
protein 4." J.Biol.Chem.,
288, 6351-6362. doi: 10.1074/jbc.M112.431098.
- Abstract
- The methyl-CpG binding domain (MBD) protein MBD4
participates in DNA repair as a glycosylase that excises
mismatched thymine bases in CpG sites and also functions in
transcriptional repression. Unlike other MBD proteins, MBD4
recognizes not only methylated CpG dinucleotides
((5m)CG/(5m)CG) but also T/G mismatched sites generated by
spontaneous deamination of 5-methylcytosine ((5m)CG/TG).
The glycosylase activity of MBD4 is also implicated in
active DNA demethylation initiated by the
deaminase-catalyzed conversion of 5-methylcytosine to
thymine. Here, we report the crystal structures of the MBD
of MBD4 (MBDMBD4) complexed with (5m)CG/(5m)CG and
(5m)CG/TG. The crystal structures show that the DNA
interface of MBD4 has flexible structural features and
harbors an extensive water network that supports its dual
base specificities. Combined with the results of
biochemical analyses, the crystal structure of MBD4 bound
to 5-hydroxymethylcytosine further demonstrates that
MBDMBD4 is able to recognize a wide range of
5-methylcytosine modifications through the unique water
network. The versatile base recognition ability of MBDMBD4
implies multifunctional roles for MBD4 in the regulation of
dynamic DNA methylation patterns coupled with deamination
and/or oxidation of 5-methylcytosine.