Summary information and primary citation
- PDB-id
-
3b58;
SNAP-derived features in text and
JSON formats
- Class
- RNA
- Method
- X-ray (2.65 Å)
- Summary
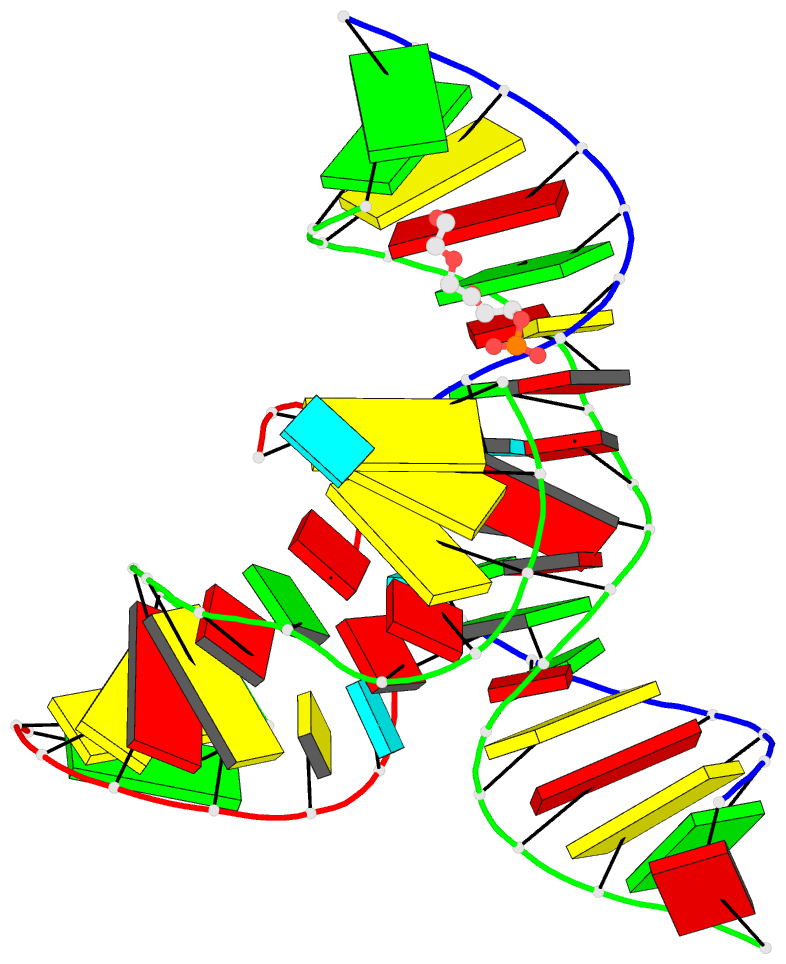
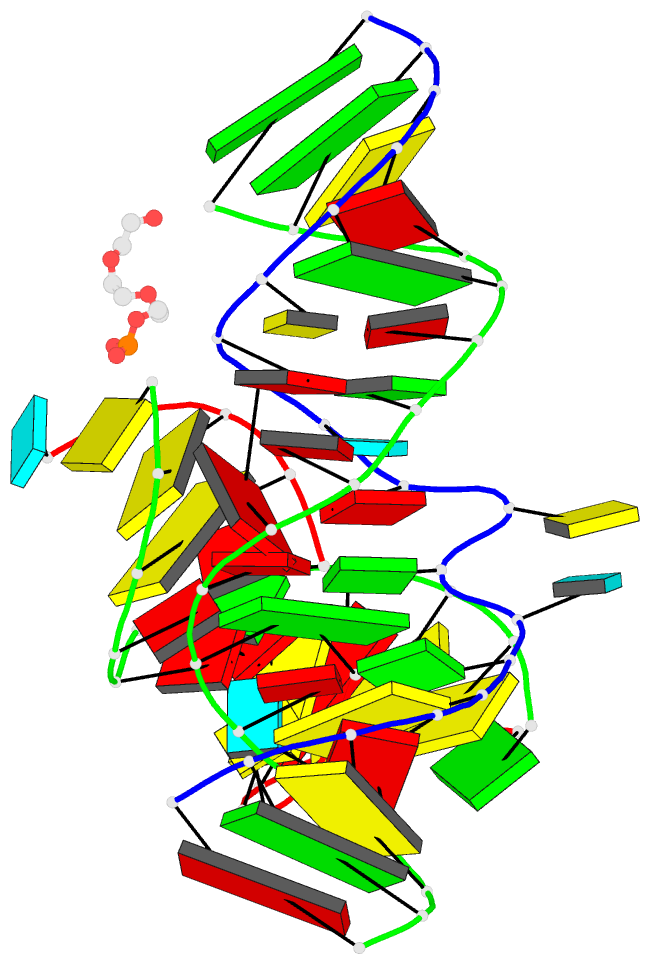
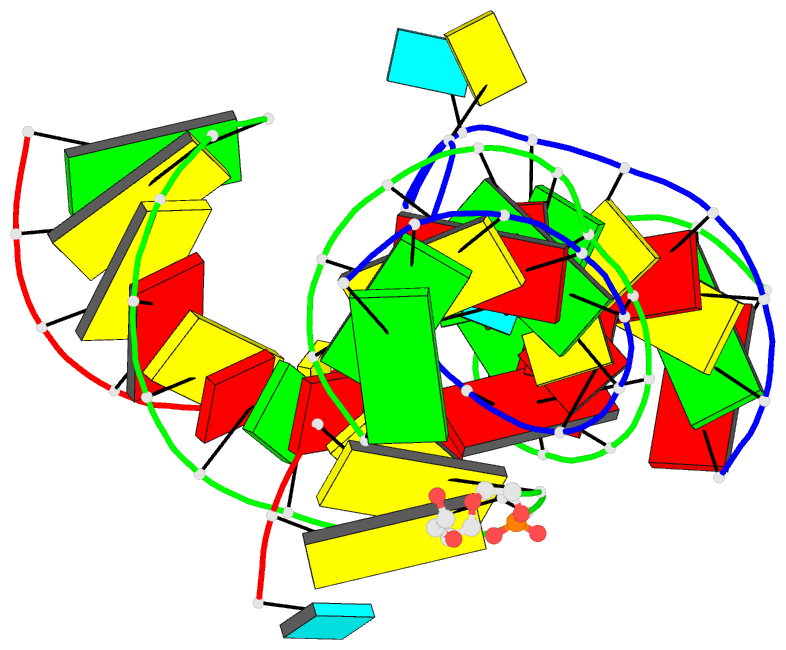
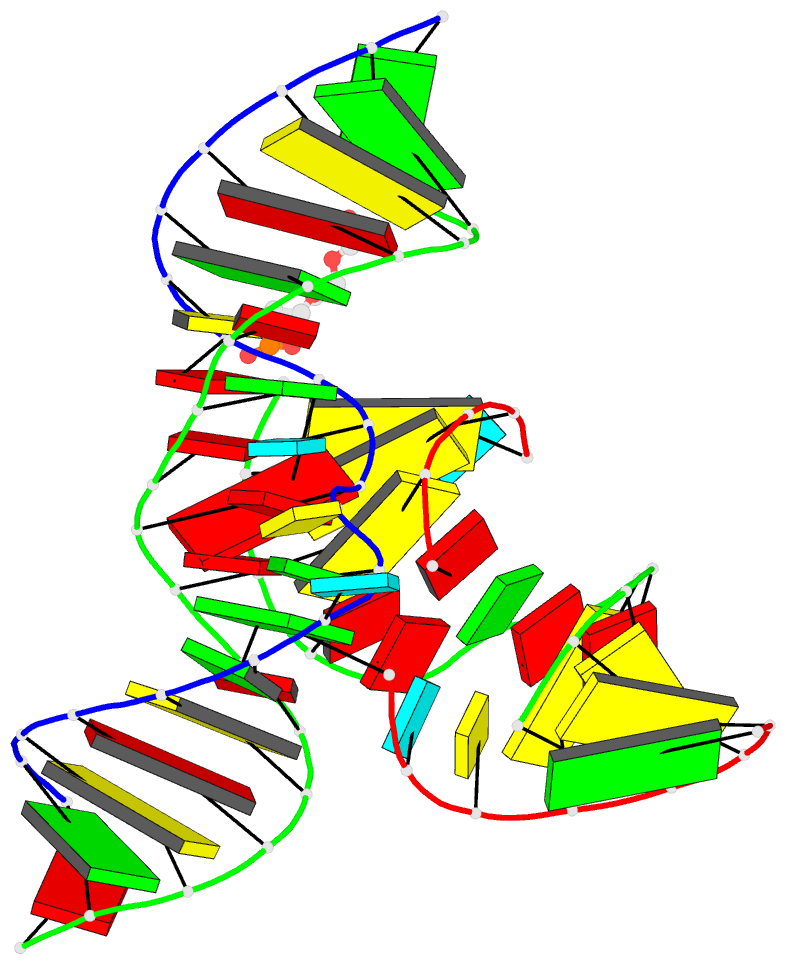
- Minimally junctioned hairpin ribozyme incorporates a38g
mutation and a 2',5'-phosphodiester linkage at the active
site
- Reference
-
MacElrevey C, Salter JD, Krucinska J, Wedekind JE (2008):
"Structural
effects of nucleobase variations at key active site
residue Ade38 in the hairpin ribozyme." Rna,
14, 1600-1616. doi: 10.1261/rna.1055308.
- Abstract
- The hairpin ribozyme requires functional groups from
Ade38 to achieve efficient bond cleavage or ligation. To
identify molecular features that contribute to catalysis,
structures of position 38 base variants 2,6-diaminopurine
(DAP), 2-aminopurine (AP), cytosine (Cyt), and guanine
(Gua) were determined between 2.2 and 2.8 A resolution. For
each variant, two substrate modifications were compared:
(1) a 2'-O-methyl-substituent at Ade-1 was used in lieu of
the nucleophile to mimic the precatalytic state, and (2) a
3'-deoxy-2',5'-phosphodiester linkage between Ade-1 and
Gua+1 was used to mimic a reaction-intermediate
conformation. While the global fold of each variant
remained intact, the results revealed the importance of
Ade38 N1 and N6 groups. Absence of N6 resulting from AP38
coincided with failure to localize the precatalytic
scissile phosphate. Cyt38 severely impaired catalysis in a
prior study, and its structures here indicated an anti base
conformation that sequesters the imino moiety from the
scissile bond. Gua38 was shown to be even more deleterious
to activity. Although the precatalytic structure was
nominally affected, the reaction-intermediate conformation
indicated a severe electrostatic clash between the Gua38
keto oxygen and the pro-Rp oxygen of the scissile bond.
Overall, position 38 modifications solved in the presence
of 2'-OMe Ade-1 deviated from in-line geometry, whereas
variants with a 2',5' linkage exhibited S-turn
destabilization, as well as base conformational changes
from syn to anti. These findings demonstrate the importance
of the Ade38 Watson-Crick face in attaining a
reaction-intermediate state and the sensitivity of the RNA
fold to restructuring when electrostatic and shape features
fail to complement.