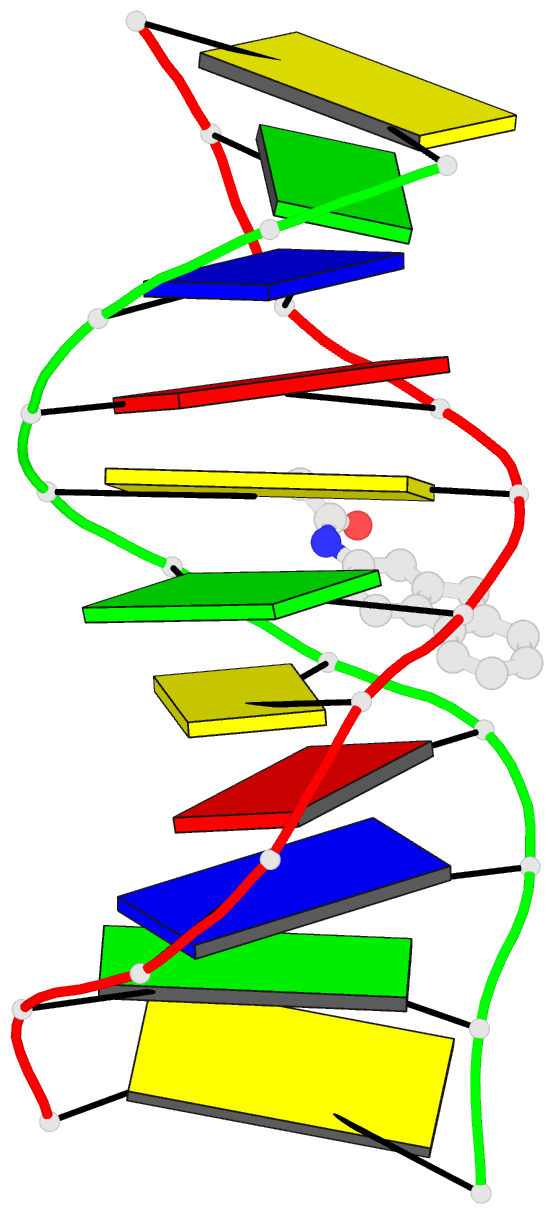
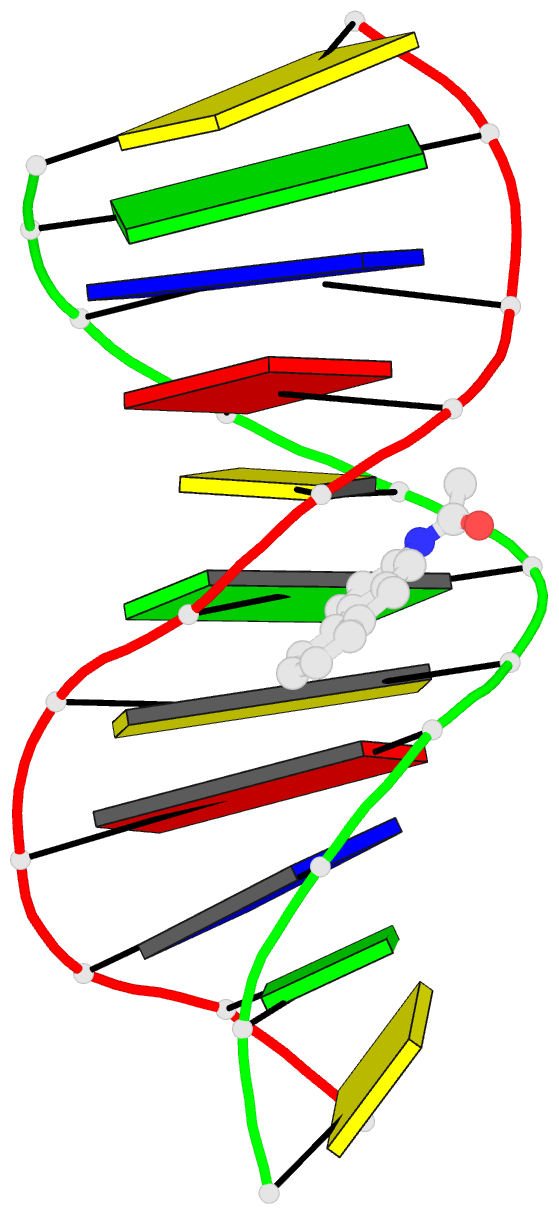
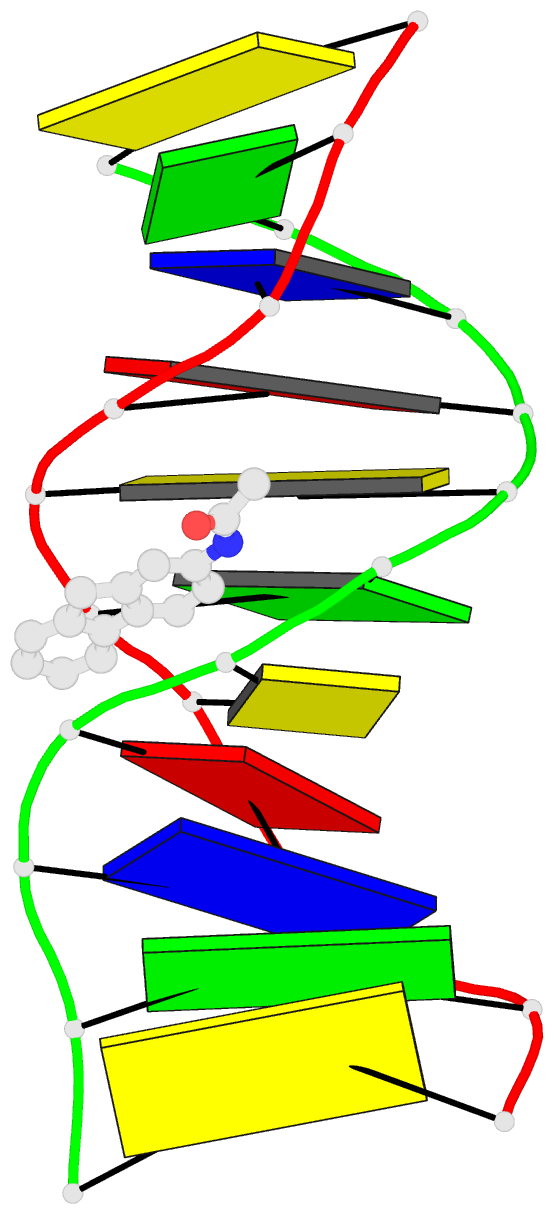
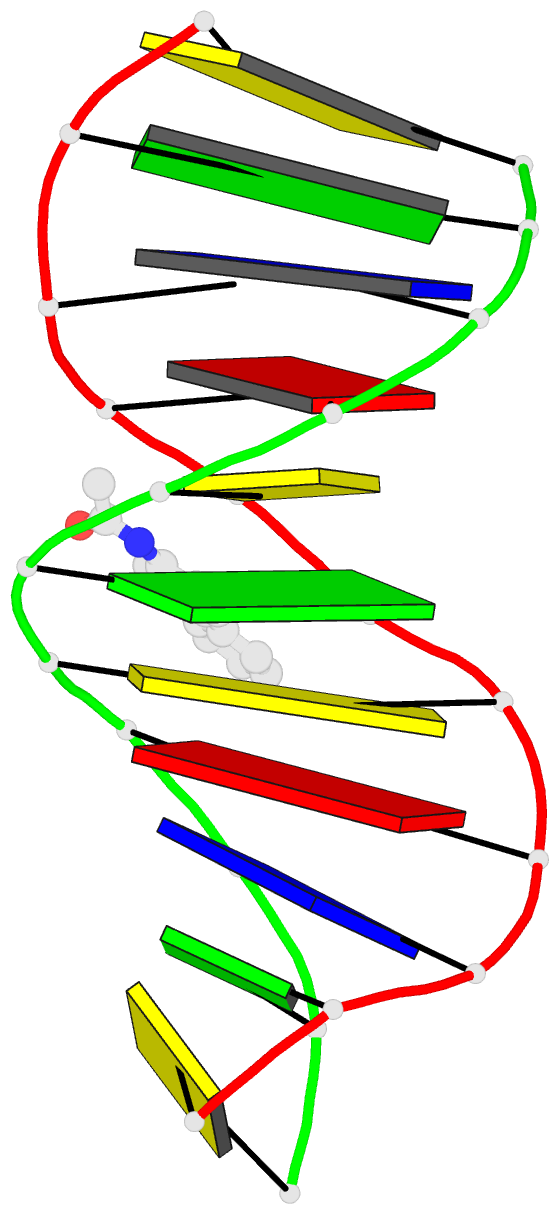
Summary information and primary citation
- PDB-id
- 2ge2; DSSR-derived features in text and JSON formats
- Class
- DNA
- Method
- NMR
- Summary
- Solution structure of the duplex DNA containing the 3-(deoxyguanosin-n2-yl)-2-acetoaminofluorene
- Reference
- Zaliznyak T, Bonala R, Johnson F, de Los Santos C (2006): "Structure and Stability of Duplex DNA Containing the 3-(Deoxyguanosin-N(2)-yl)-2-acetylaminofluorene (dG(N(2))-AAF) Lesion: A Bulky Adduct that Persists in Cellular DNA." Chem.Res.Toxicol., 19, 745-752. doi: 10.1021/tx060002i.
- Abstract
- The carcinogenic environmental pollutant 2-nitrofluorene produces several DNA adducts including the minor 3-(deoxyguanosin-N(2)-yl)-2-acetylaminofluorene (dG(N(2))-AAF) lesion, which persists for long times in rat tissue DNA after discontinuation of carcinogen administration. Here, we present the solution structure of a dG(N(2))-AAF duplex as determined by NMR spectroscopy and restrained molecular dynamics. The data establish a regular right-handed conformation with Watson-Crick base pair alignments throughout the duplex. The AAF moiety resides in the minor grove of the helix with its long axis directed toward the 5'-end of the modified strand. Restrained molecular dynamics shows that the duplex structure adjusts to the AAF lesion, reducing its exposure to water molecules. Analysis of UV melting profiles shows that the presence of dG(N(2))-AAF increases the thermal and thermodynamic stability of duplex DNA, an effect that is driven by a favorable entropy. The structure and stability of the dG(N(2))-AAF duplex have important implications in understanding the recognition of bulky lesions by the DNA repair system.