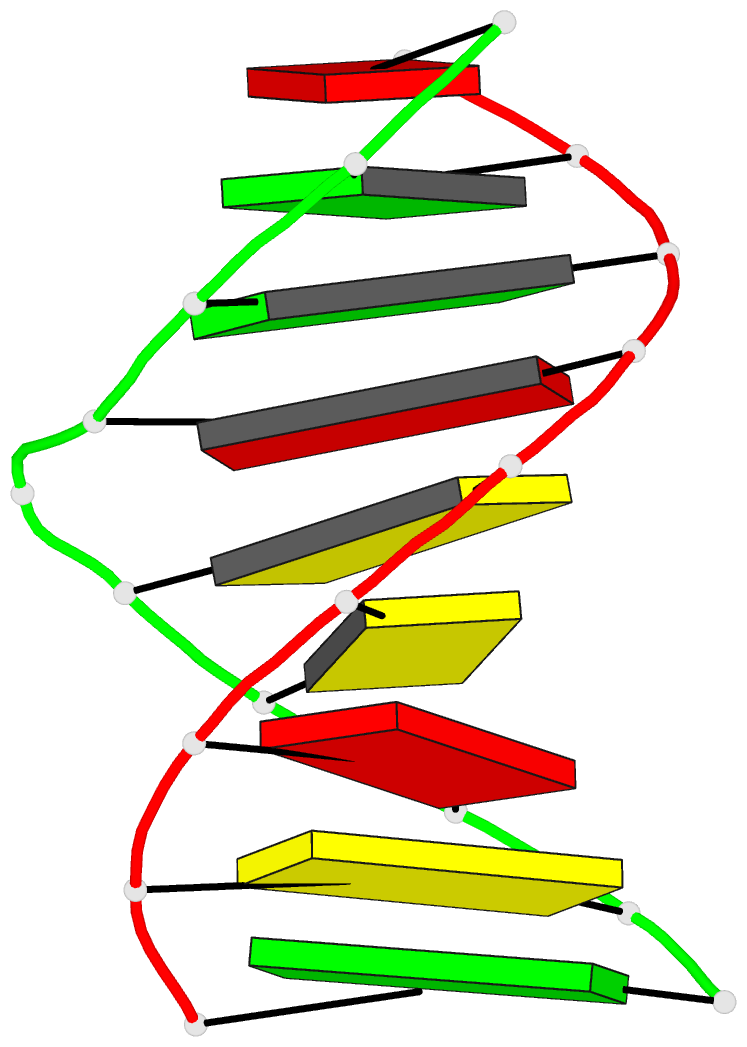
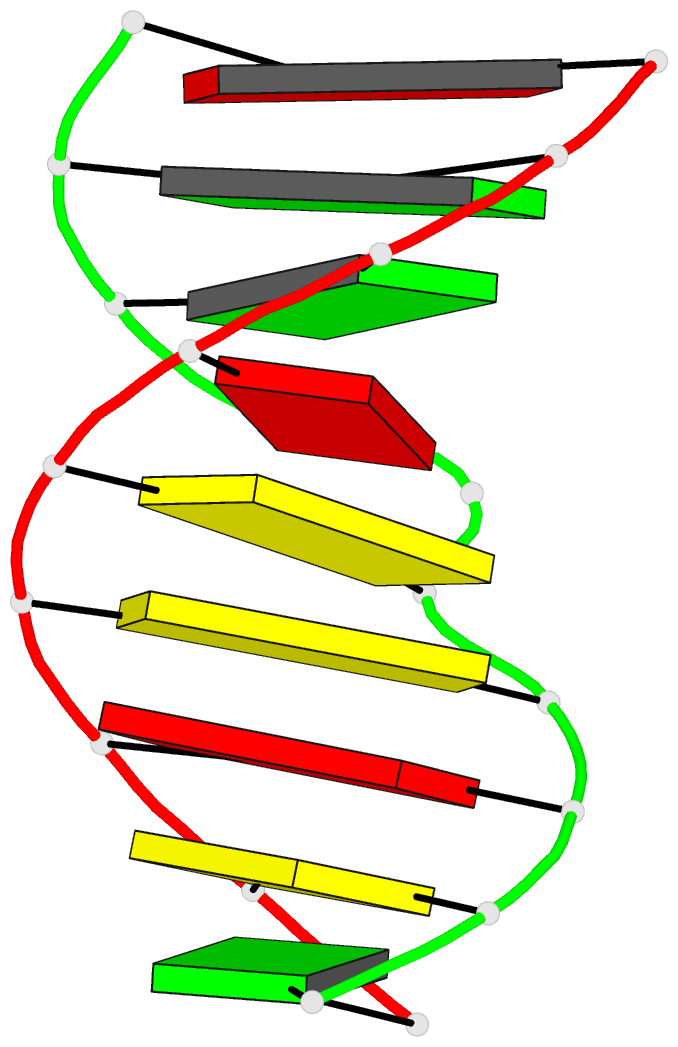
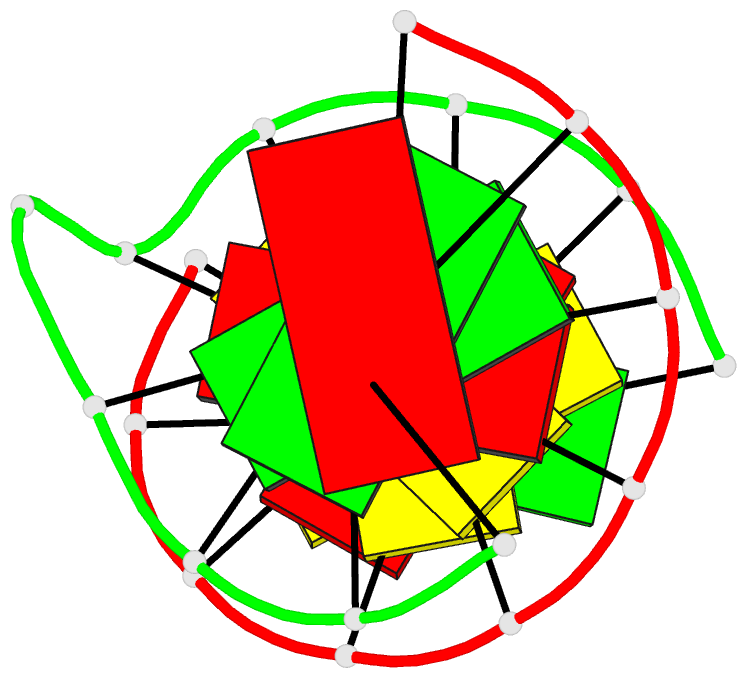
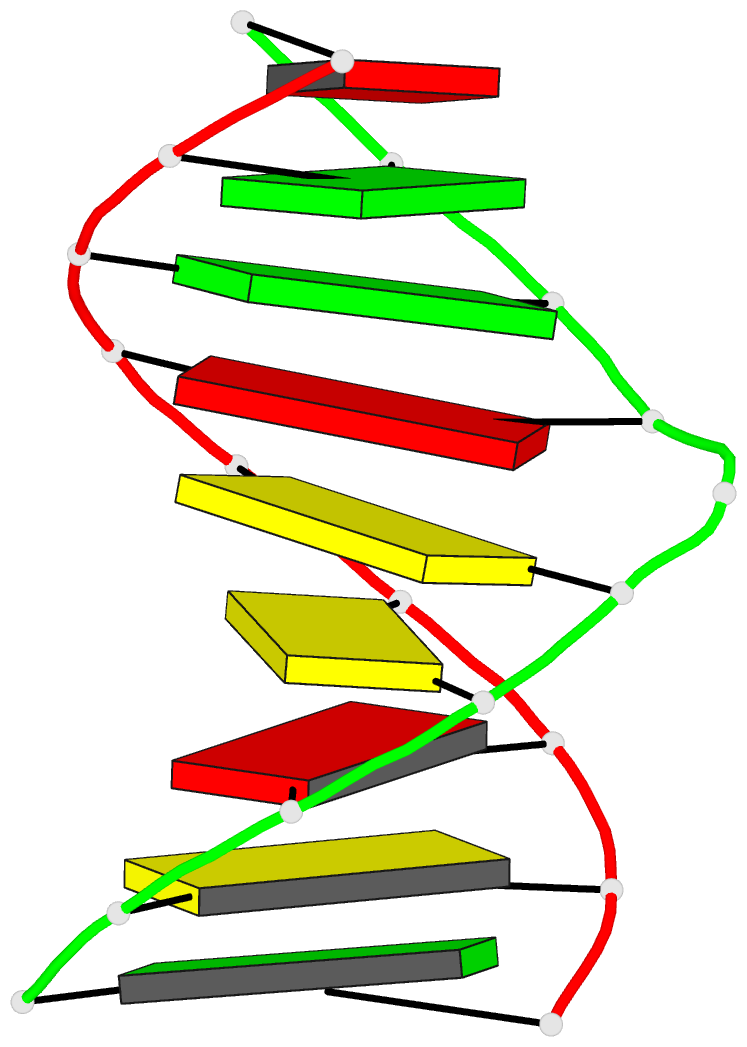
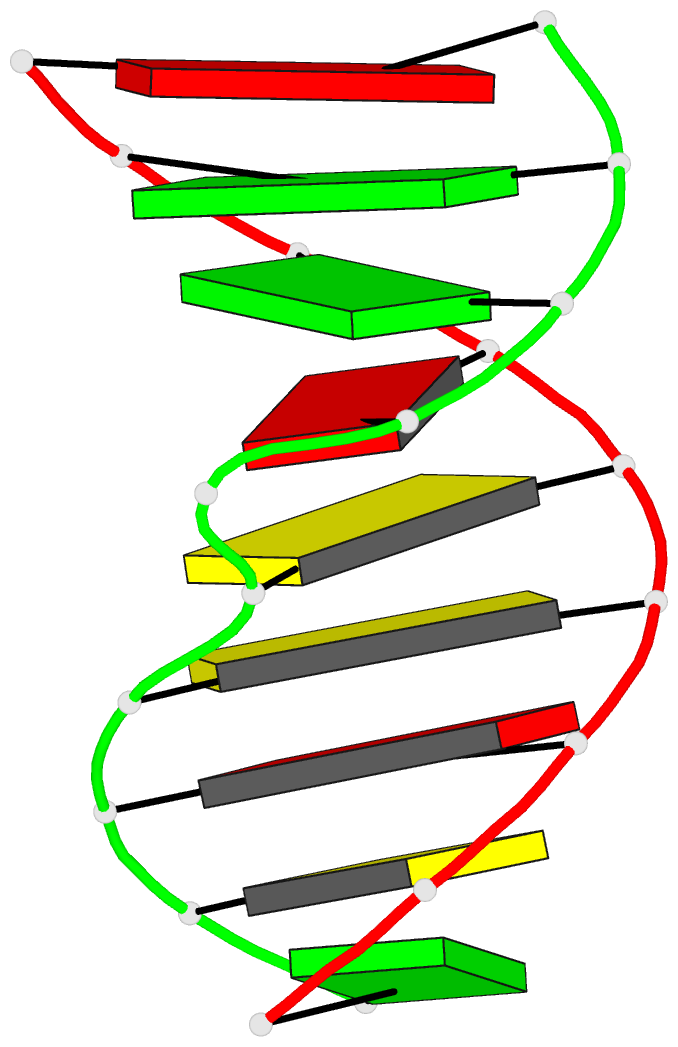
Summary information and primary citation
- PDB-id
-
1osr;
SNAP-derived features in text and
JSON formats
- Class
- DNA
- Method
- NMR
- Summary
- Structural study of DNA duplex containaing a
n-(2-deoxy-beta-erytho-pentofuranosyl) formamide frameshift
by NMR and restrained molecular dynamics
- Reference
-
Maufrais C, Fazakerley GV, Cadet J, Boulard Y (2003):
"Structural
study of DNA duplex containing an
N-(2-deoxy-beta-D-erythro-pentofuranosyl) formamide
frameshift by NMR and restrained molecular dynamics."
Nucleic Acids Res., 31,
5930-5940. doi: 10.1093/nar/gkg803.
- Abstract
- The presence of an
N-(2-deoxy-beta-D-erythro-pentofuranosyl) formamide (F)
residue, a ring fragmentation product of thymine, in a
frameshift context in the sequence
5'-d-(AGGACCACG)*d(CGTGGFTCCT) has been studied by 1H and
31P nuclear magnetic resonance (NMR) and molecular
dynamics. Two-dimensional NMR studies show that the
formamide residue, whether the cis or trans isomer, is
rotated out of the helix and that the bases on either side
of the formamide residue in the sequence, G14 and T16, are
stacked over each other in a way similar to normal B-DNA.
The cis and trans isomers were observed in the ratio 3:2 in
solution. Information extracted from 31P NMR data reveal a
modification of the phosphodiester backbone conformation at
the extrahelical site, which is also observed during the
molecular dynamics simulations.