Summary information and primary citation
- PDB-id
-
1bbx;
SNAP-derived features in text and
JSON formats
- Class
- DNA binding protein-DNA
- Method
- NMR
- Summary
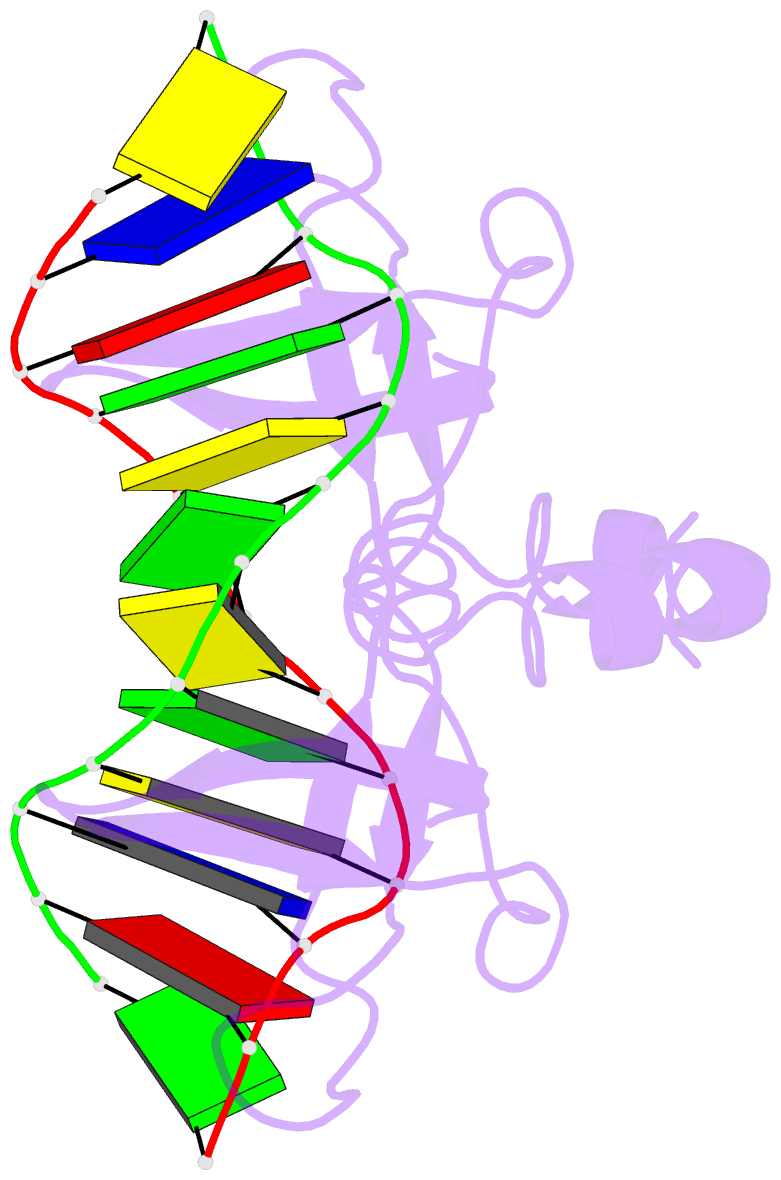
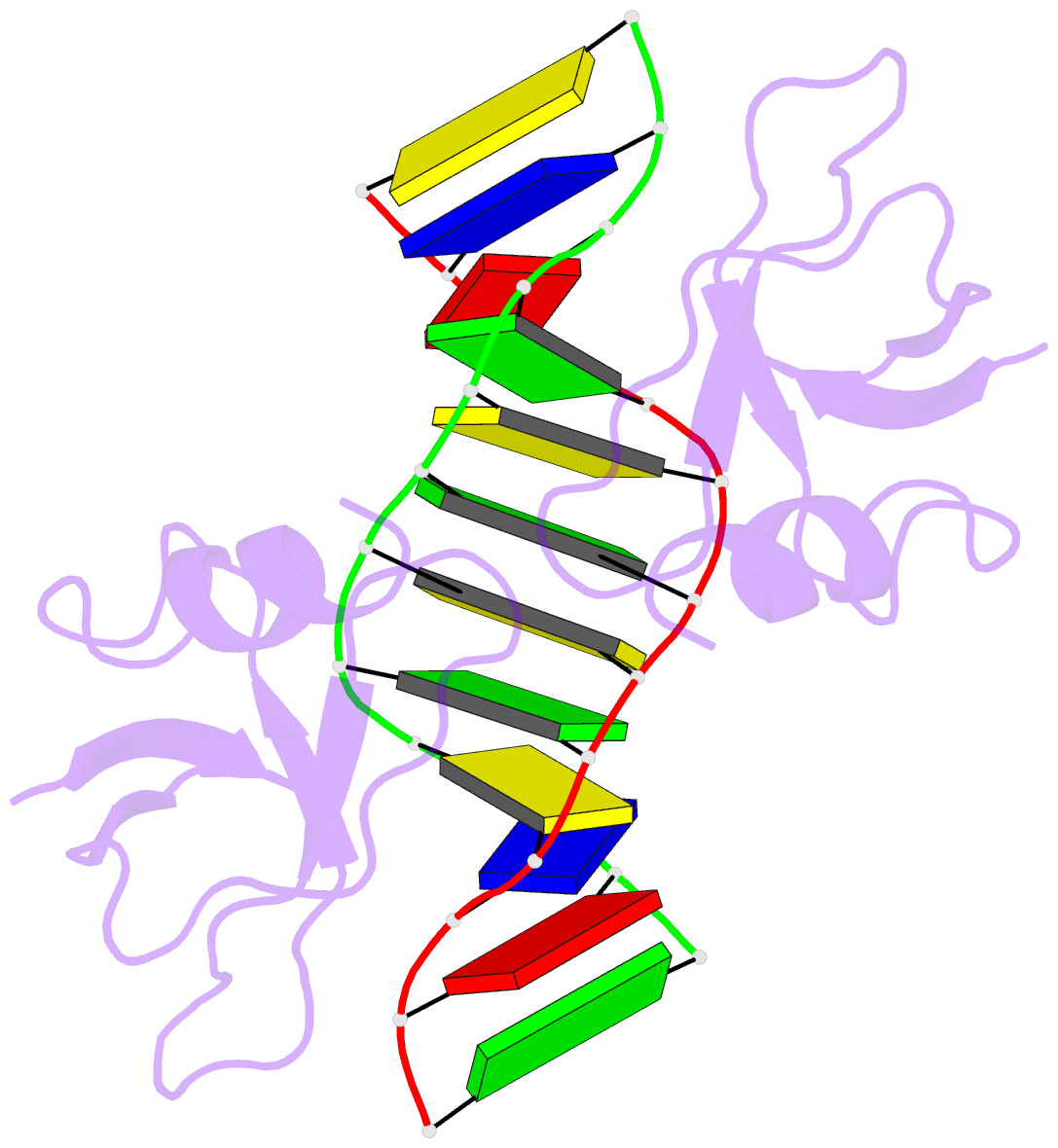
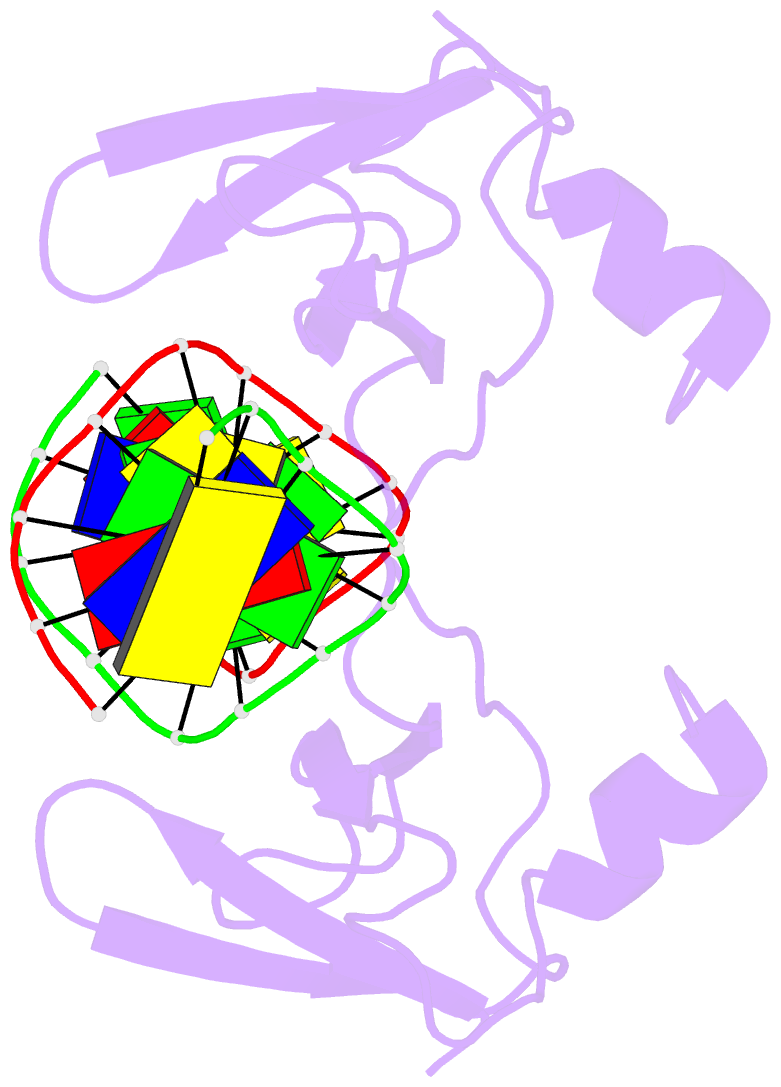
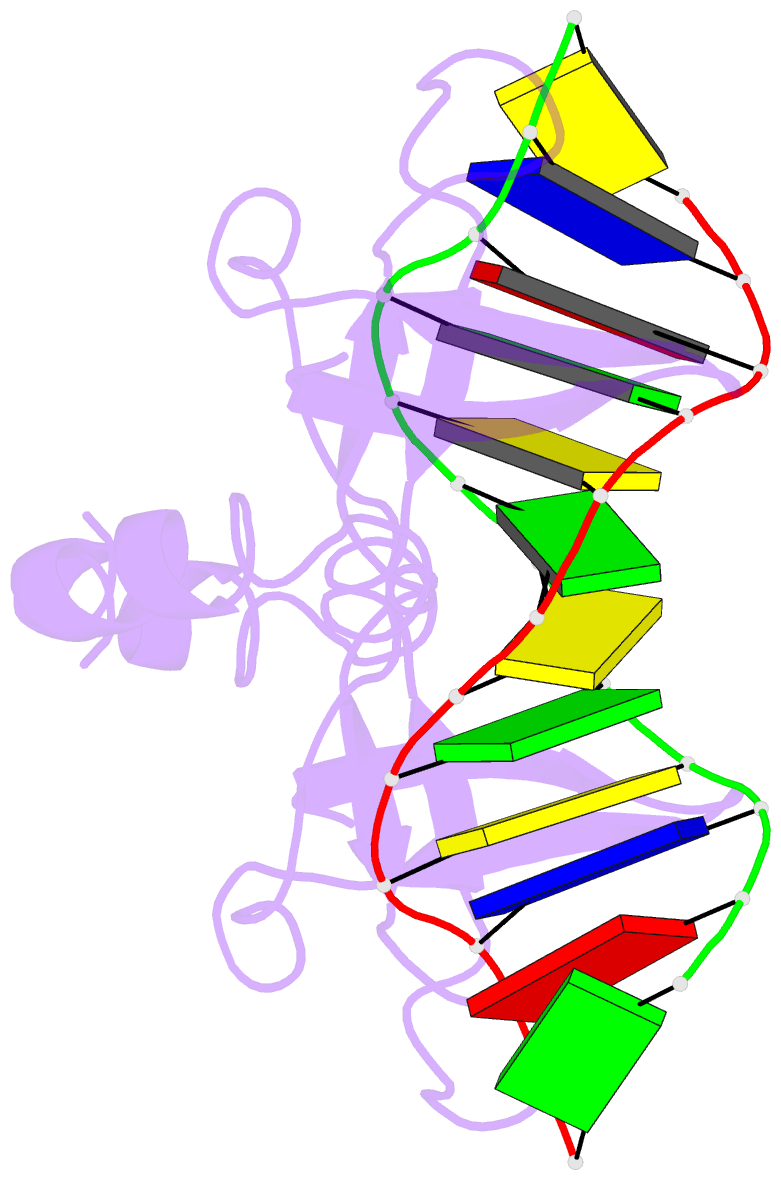
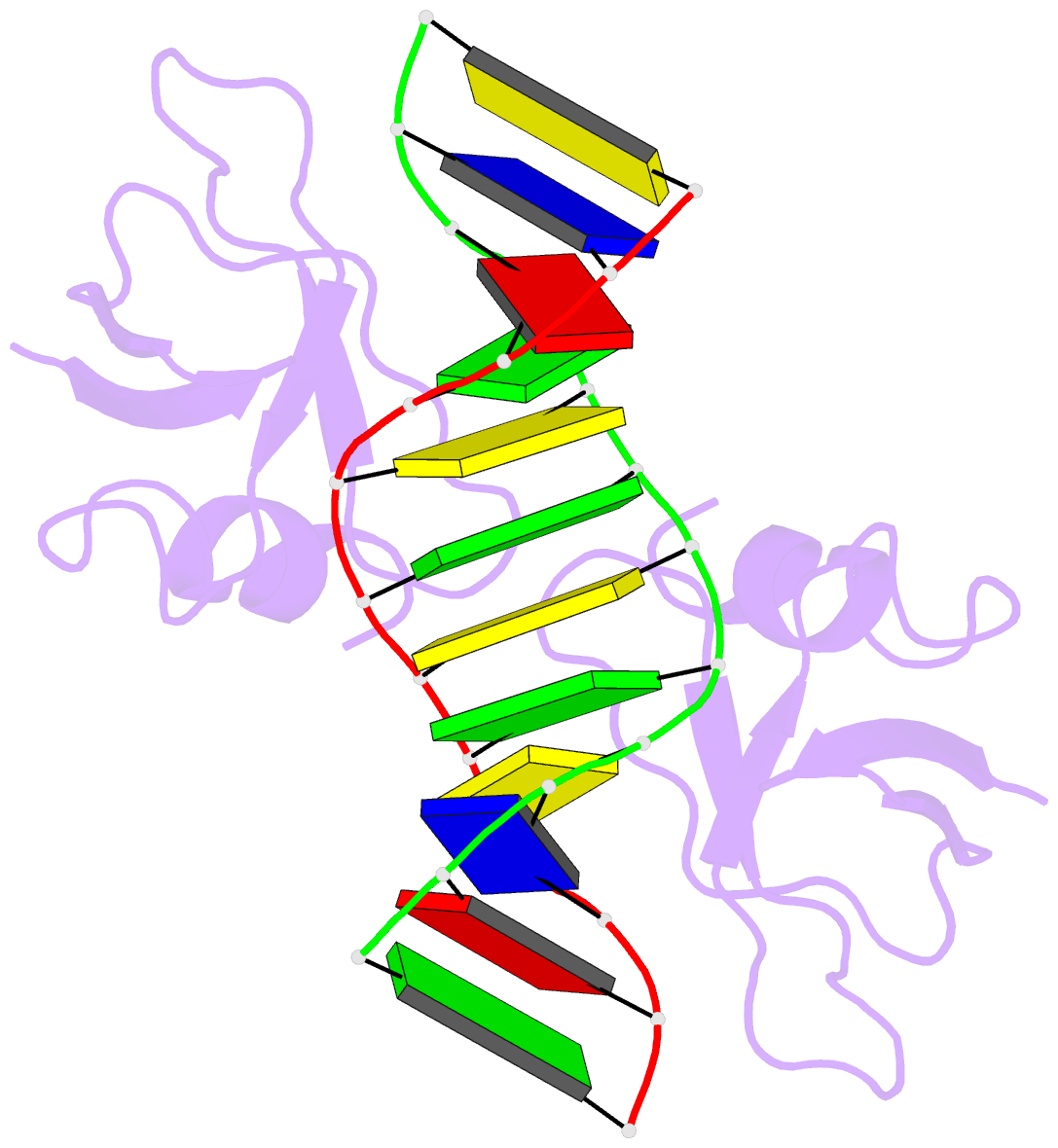
- Non-specific protein-DNA interactions in the sso7d-DNA
complex, NMR, 1 structure
- Reference
-
Agback P, Baumann H, Knapp S, Ladenstein R, Hard T
(1998): "Architecture
of Nonspecific Protein-DNA Interactions in the Sso7D-DNA
Complex." Nat.Struct.Biol.,
5, 579-584. doi: 10.1038/836.
- Abstract
- Many biochemical processes, including DNA packing,
maintenance and control, rely on non-sequence specific
protein-DNA interactions. Nonspecific DNA-binding proteins
have evolved to tolerate a wide range of DNA sequences, yet
bind with a respectable affinity. The nonspecific binding
requirement is in contrast to that imposed on, for example,
transcription factors and implies a different structural
basis for the biomolecular recognition process. To address
this issue, and the mechanism for archaeal DNA packing, we
determined the structure of the Sso7d protein from
Sulfolobus solfataricus in complex with DNA. Sso7d binds
DNA by placing a triple-stranded beta-sheet across the DNA
minor groove. The protein is anchored in this position by
the insertion of hydrogen bond-donating side chains into
the groove and additionally stabilized by electrostatic and
non-polar interactions with the DNA backbone. This
structure explains how strong binding can be achieved
independent of DNA sequence. Sso7d binding also distorts
the DNA conformation and introduces significant unwinding
of the helix. This effect suggests a mechanism for DNA
packing in Sulfolobus based on negative DNA
supercoiling.