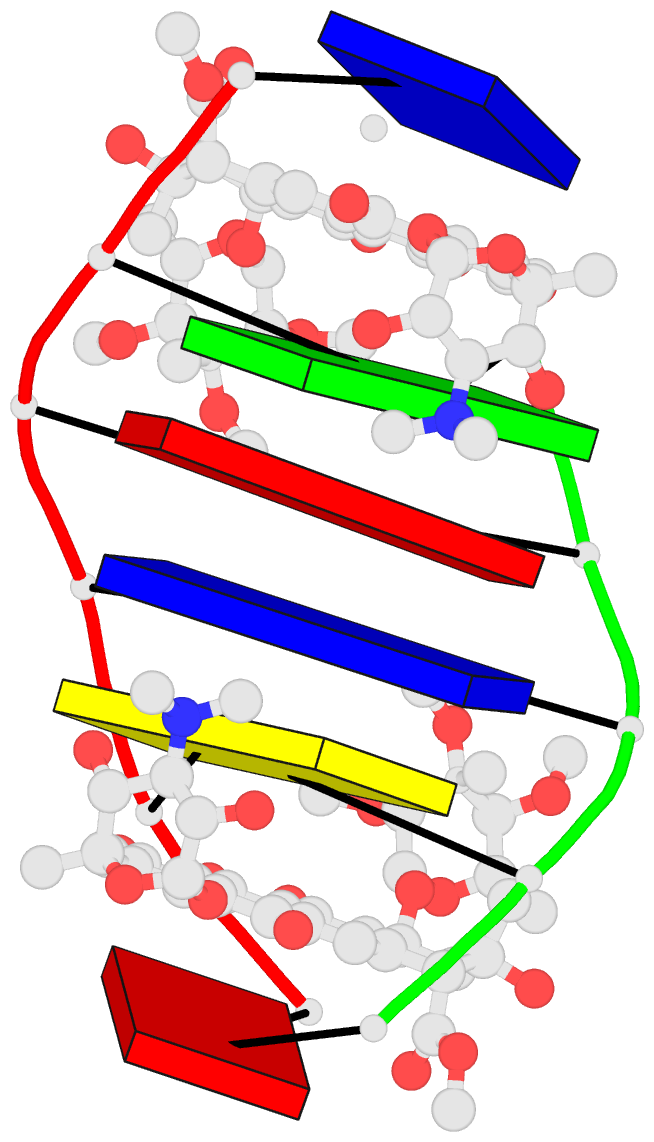
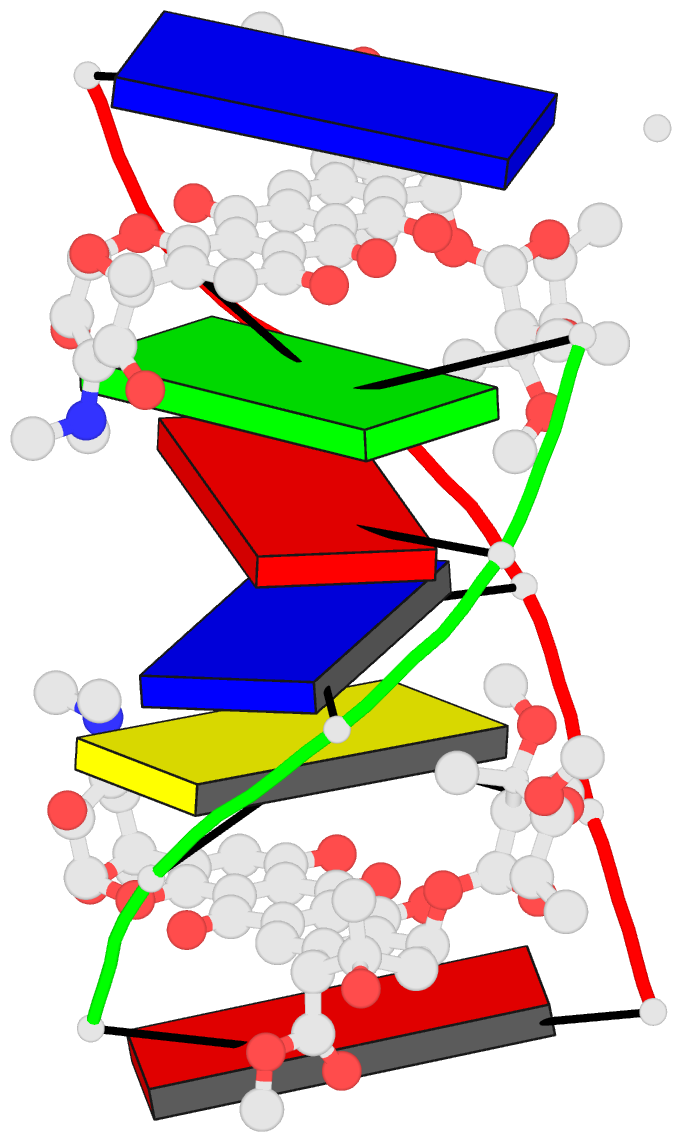
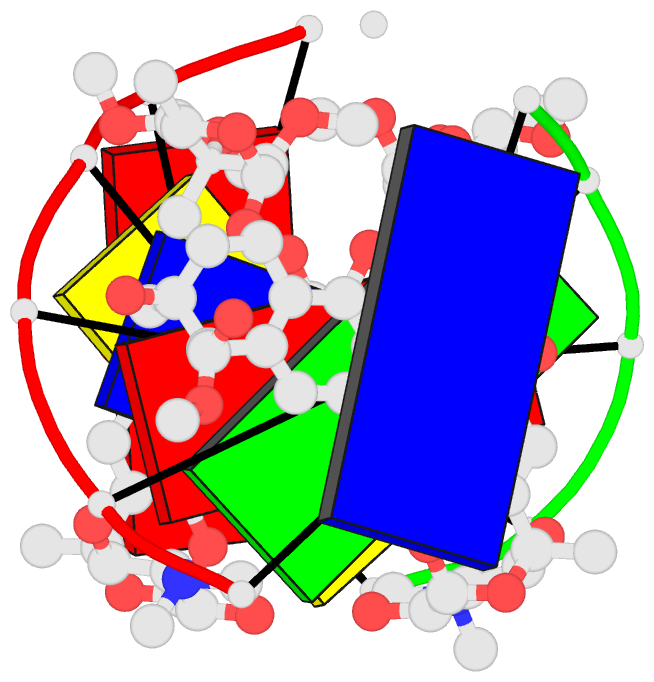
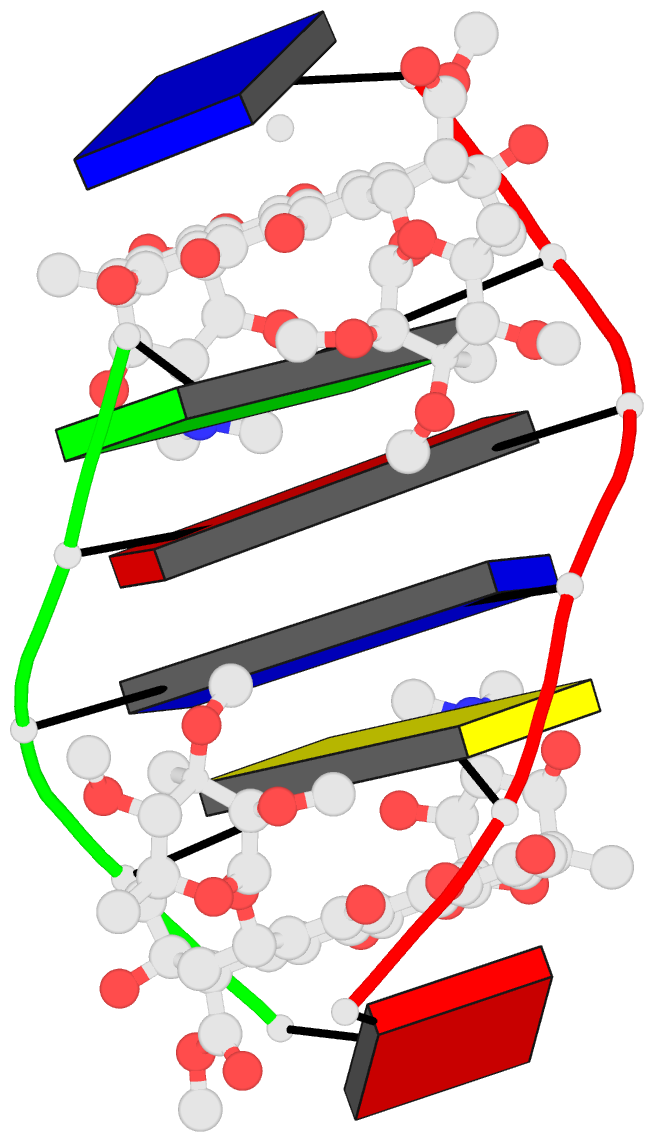
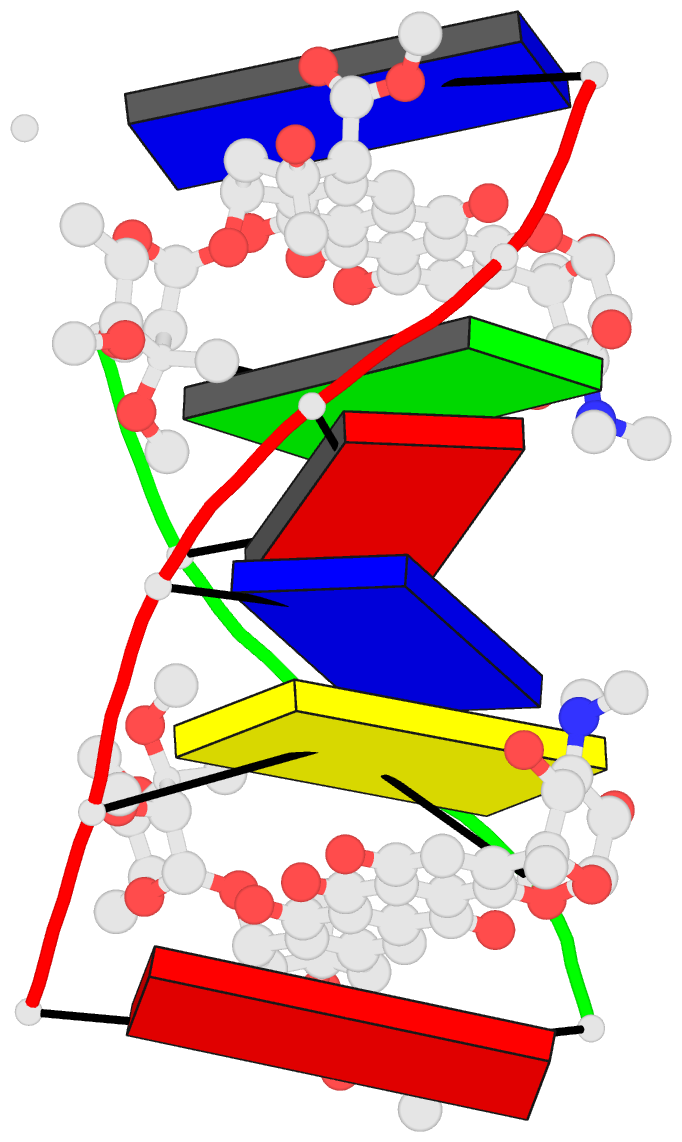
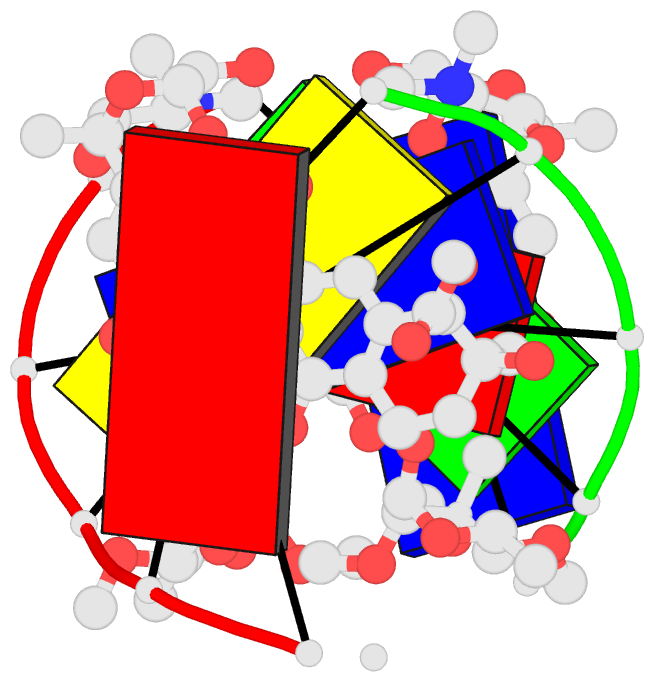
Summary information and primary citation
- PDB-id
-
182d;
SNAP-derived features in text and
JSON formats
- Class
- DNA
- Method
- X-ray (1.8 Å)
- Summary
- DNA-nogalamycin interactions: the crystal structure of
d(tgatca) complexed with nogalamycin
- Reference
-
Smith CK, Davies GJ, Dodson EJ, Moore MH (1995):
"DNA-nogalamycin
interactions: the crystal structure of d(TGATCA)
complexed with nogalamycin." Biochemistry,
34, 415-425. doi: 10.1021/bi00002a005.
- Abstract
- The structure of the self-complementary
deoxyoligonucleotide d5'(TGATCA) complexed with
nogalamycin, an antitumor anthracycline, has been solved to
1.8 A resolution using X-ray crystallographic methods. The
technique of single isomorphous replacement, utilizing the
anomalous signal of bromine in derivative data collected at
three different wavelengths, Cu K alpha, Mo K alpha, and
0.91 A synchroton radiation, was used. The complex
crystallized in space group P4(1)2(1)2 with unit cell
dimensions a = 37.2 A and c = 70.1 A. The final structure
including 116 water molecules has an overall R factor of
19.5% for the 4767 reflections with F > or = 1 sigma F
in the resolution range 10.0-1.8 A. One nogalamycin
molecule intercalates between each of the d5'(TpG) steps at
both ends of a distorted B DNA double helix. This structure
provides the first three-dimensional picture of nogalamycin
bound to the triplet sequence d5'(TGA), one of its
favorable natural binding sites. The drug exhibits a strict
requirement for binding to the 3' side of a pyrimidine and
the 5' side of a purine. Nogalamycin has bulky sugar groups
at either end of a planar aglycon chromophore; therefore,
in order for intercalation to occur, the DNA must either
transiently open or flex along the helix axis to allow
insertion of the chromophore between the base pairs.
Conformational change in nogalamycin is observed in the
drug-DNA complex with respect to free nogalamycin.
Nogalamycin binding to DNA induces severe deformation to
the intercalation site base pairs. In comparison to
previously reported anthracycline-DNA structures
significant differences in base-pair geometry, drug
hydrogen-bonding patterns, and the extent of hydration are
observed. The position of the drug in this complex is
stabilized by a number of nonbonded forces including van
der Waals interactions and extensive direct and
solvent-mediated hydrogen bonds to the DNA duplex.